☐ | Preliminary Proxy Statement |
☐ | Confidential, for Use of the Commission Only (as permitted by Rule |
☒ | Definitive Proxy Statement |
☐ | Definitive Additional Materials |
☐ | Soliciting Material Pursuant to Section 240.14a-12 |

☒ | No fee required. |
☐ | Fee paid previously with preliminary materials. |
☐ | Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and0-11 |




| ÷ ÷ ÷ ÷ | 1 |
Notice of Annual Meeting
of Shareholders
To Merck Shareholders:
You are invited to the Annual Meeting of Shareholders of Merck & Co., Inc. (the “Company” or “Merck”) on Tuesday, May 23, 2023,28, 2024, at 9:00 a.m. (Eastern Time) via Webcast at www.virtualshareholdermeeting.com/MRK2023.MRK2024 (the “2024 Annual Meeting”).
The purposes of the meeting are to:
1. Elect the 1312 Director nominees named in this proxy statement;
2. Consider and act upon a proposal to approve, by non-binding advisory vote, the compensation of our Named Executive Officers;
3. Consider and act upon a proposal to approve, by non-binding advisory vote, the frequency of future votes to approve the compensation of our Named Executive Officers;
4. Consider and act upon a proposal to ratify the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for 2023;2024;
4. Consider and act upon a shareholder proposal regarding a shareholder right to act by written consent, if properly presented at the meeting;
5. Consider and act upon a shareholder proposal regarding business operations in China,a government censorship transparency report, if properly presented at the meeting;
6. Consider and act upon a shareholder proposal regarding access to COVID-19 products, if properly presented at the meeting;
7. Consider and act upon a shareholder proposal regarding indirect political spending, if properly presented at the meeting;
8. Consider and act upon a shareholder proposal regarding patents and access, if properly presented at the meeting;
9. Consider and act upon a shareholder proposal regarding a congruency report of partnerships with globalist organizations, if properly presented at the meeting;
10. Consider and act upon a shareholder proposal regarding an independent board chairman,on respecting workforce civil liberties, if properly presented at the meeting; and
11.7. Transact such other business as may properly come before the meeting.
By order of the Board of Directors,
Kelly E. W. Grez
Corporate Secretary
Vote Right Away—Advance voting methods and deadlines
We encourage all shareholders of record to read this proxy statement with care and vote right away using any of the following methods, even if they intend to attend the 2024 Annual Meeting. In all cases, have your proxy card or voting instruction form in hand and follow the instructions.
| ||||
 | BY INTERNET* | www.proxyvote.com | ||
 | BY PHONE* | In the U.S. or Canada dial toll-free 1-800-690-6903 | ||
  | BY QR CODE |
| ||
 | BY MAIL** | Cast your ballot, sign your proxy card and send in our prepaid envelope | ||
Only shareholders listed on the Company’s records at the close of business on
Merck began distributing its Notice of Internet Availability of Proxy Materials, proxy statement, the
IMPORTANT NOTICE REGARDING THE AVAILABILITY OF PROXY MATERIALS FOR THE ANNUAL MEETING OF SHAREHOLDERS TO BE HELD ON
The Notice of Annual Meeting of Shareholders, proxy statement and the The principal executive offices of the Company are located at 126 East Lincoln Avenue, Rahway, N.J. 07065 U.S.A.
* The telephone and internet voting facilities will close at 11:59 p.m. Eastern Time on May ** You will need the 16-digit control number included on your proxy card, voting instruction form or Notice of Internet Availability of Proxy Materials. If your shares are held in a stock brokerage account or by a bank or other nominee, your ability to vote by telephone or over the internet depends on your broker’s voting process. Please follow the directions provided to you by your broker, bank or nominee.
| ||||
Merck & Co., Inc. 2024 Proxy Statement
| 2 | ç ç ç ç |
Dear Merck Shareholders,
2023 was a very strong year for our company as we continued to deliver for all our stakeholders, including patients, society and you, our shareholders. Driven by our purpose of using the power of leading-edge science to save and improve lives around the world, our team worked with urgency, rigor and passion to develop and deliver important scientific advancements. I am confident our science-led strategy, which keeps the patient at the center of everything we do, will enable us to continue driving value creation today and well into the next decade.
Over the last year, we continued to advance our priority programs and took meaningful steps to build one of the broadest and most diversified pipelines in our recent history. We initiated more than 20 Phase 3 studies, which included advancing eight novel candidates. In 2024, we anticipate an even greater number of Phase 3 study starts and multiple data readouts. We also look forward to three potential new product approvals with the promise to provide significant patient benefit.
In oncology, September 2024 marks a decade since the first approval of KEYTRUDA, a foundational medicine for the treatment of certain types of cancer, currently approved by the U.S. Food and Drug Administration (FDA) for 39 indications across 17 different tumor types. We are committed to continuing to provide important innovation to patients and maintaining our leadership in oncology. Our strong, diverse oncology pipeline includes candidates spanning immuno-oncology, precision molecular targeting and tissue-targeting agents.
As we continue to realize the potential of KEYTRUDA, we are focusing increasingly on earlier stages of disease, where effective intervention has the potential to improve outcomes. We made progress in 2023 for the treatment of certain patients with non-small cell lung cancer (NSCLC) with FDA approvals for KEYNOTE-091 as well as KEYNOTE-671, which met its dual primary endpoints of event-free survival and overall survival. In January 2024, we announced FDA approval for KEYTRUDA in combination with
“I am confident our science-led strategy, which keeps the patient at the center of everything we do, will enable us to continue driving value creation today and well into the next decade.”
chemoradiotherapy for the treatment of stage III through IVA high-risk, locally advanced cervical cancer, based on the Phase 3 KEYNOTE A-18 trial. In the earlier-stage setting, we and our partner Moderna announced positive three-year recurrent-free survival and distant metastasis-free survival data for our individualized neoantigen therapy, V940, in combination with KEYTRUDA for the adjuvant treatment of certain patients with stage III and IV melanoma following complete resection. Together with collaborators, we also announced FDA approval for KEYTRUDA in combination with Padcev, a treatment for adult patients with locally advanced or metastatic urothelial cancer.
We’re making progress with our precision molecular targeting and tissue targeting oncology molecules. The FDA approval of an additional indication for WELIREG, our HIF-2a inhibitor for the treatment of adults with advanced renal cell carcinoma following a PD-1 or PD-L1 inhibitor and a VEGF-TKI, marks the first approval in a novel therapeutic class for this population in nearly a decade. In addition, we showed meaningful progress in our robust oncology pipeline, initiating Phase 3 trials for four investigational medicines, including bomedemstat (LSD1 inhibitor), nemtabrutinib (BTK inhibitor), MK-2870 (anti-TROP2 antibody-drug conjugate, or ADC) and MK-5684 (CYP11A1 inhibitor). We have built a pipeline of ADCs through our collaborations with Kelun-Biotech and Daiichi Sankyo as well as our own discovery programs.
Our growing cardiometabolic pipeline is another exciting area of focus, one where we see significant long-term potential. We recently received FDA approval for WINREVAIR (sotatercept-csrk), a first-in-class activin signaling inhibitor biologic for the treatment of adults with pulmonary arterial hypertension (PAH). PAH is a rare, progressive and potentially devastating disease of the blood vessels in the lungs, which has a profound impact on patients’ lives and on their life expectancy. Additionally, we are working to bring this important medicine to patients in the European Union, where we expect regulatory action in the second half of 2024.
Beyond WINREVAIR, promising candidates like MK-0616, a novel oral PCSK9 inhibitor for the treatment of hypercholesterolemia currently in Phase 3 trials, and MK-6024, an investigational GLP-1/glucagon receptor co-antagonist being evaluated for the treatment of metabolic dysfunction-associated steatohepatitis (MASH), currently in Phase 2 trials, reinforce our expectation that we can achieve positive impact for patients and the potential for approximately $15 billion in revenue from medicines to treat cardiometabolic disease by the mid-2030s.
In our vaccines business, worldwide demand for our vaccines for the prevention of certain human papillomavirus (HPV)-related cervical cancers and other diseases – GARDASIL
Merck & Co., Inc. 20232024 Proxy Statement
Dear Merck Shareholders,
|
| |||||
was studied as an add-on to PAH background therapy. We’ve also received encouraging results from the Phase 2b trial evaluating MK-0616, an oral PCSK9 inhibitor for the treatment of hypercholesterolemia. As a result of these positive data, we plan to move to Phase 3 in the second half of this year. Our progress with sotatercept and MK-0616 is also supplemented by strong momentum in other strategic areas including our Factor XI inhibitor, MK-2060, which received fast-track designation from the U.S. Food and Drug Administration (FDA) in August. Based on these most recent data readouts and the progress we’ve made across our pipeline, we are even more confident in our potential to achieve greater than $10 billion in revenue from our cardiovascular program by the mid-2030s.
Oncology products KEYTRUDA, Lenvima, Lynparza and WELIREG are driving key growth for our business, and we remain focused on our expansive oncology research efforts, including moving to earlier stages of disease where there is a higher probability of achieving improved long-term outcomes for patients. In 2022, we continued our momentum, with approvals for KEYTRUDA and Lynparza in additional tumor types and earlier-stage cancers.
In collaboration with Moderna, we announced that KEYTRUDA in combination with mRNA4157/V940, a personalized mRNA therapeutic cancer vaccine, received breakthrough therapy designation from the FDA for adjuvant treatment of certain patients with high-risk melanoma. This follows results demonstrating statistically significant recurrence-free survival in the Phase 2b trial studying the combination, the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial. We believe this combination has great potential for patients. We also recently received approval for KEYTRUDA for adjuvant treatment after resection and platinum-based chemotherapy for certain adult patients with non-small cell lung cancer based on the results of KEYNOTE-091, which represents its seventh indication in earlier-stage cancers. Early lung cancer detection and screening remain an important unmet need. It is our ambition, along with stakeholders, to improve lung cancer screening rates to levels similar to other cancer types, such as breast and colon, where screening programs have been more widely adopted.
We have made substantial progress in advancing our oncology program through our internal R&D and business development, and we believe that our suite of antibody-drug conjugates and small molecules represent greater than $10 billion of commercial potential by the mid-2030s. This excludes additional innovation we are investing in to expand, deepen and extend our current commercial assets, and does not include the personalized mRNA therapeutic cancer vaccine.
Our vaccines business remains a key growth pillar, anchored by our HPV vaccines GARDASIL and GARDASIL 9. We expect our HPV vaccines to continue growing substantially, potentially more than doubling 2021 sales and generating over $11 billion
Merck & Co., Inc. 2023 Proxy Statement
| ÷ ÷ ÷ ÷ | 3 |
and GARDASIL 9 – continues to grow. We are proud of our legacy and leadership in revenue by 2030. Additionally,the prevention of certain HPV-related cancers and diseases, and we are advancingexpect to continue to protect millions more people around the world. Also in vaccines, the FDA granted Priority Review for our population-specific strategy in pneumococcal vaccines. VAXNEUVANCE isbiologics license application for V116. If approved, in infants, children and adultsV116 would be the first vaccine specifically designed to address the serotypes responsible for protection againstapproximately 83% of invasive pneumococcal disease in adults ages 65 and later this year, we look forward to completingolder. We anticipate launching V116 in the Phase 3 clinical trials from our V116 program for the protectionsecond half of adults against pneumococcal disease. In December, Instituto Butantan in Brazil – an organization2024, and we are collaborating with for vaccine development – reported encouraging topline results for its vaccine candidate to prevent dengue, which will help inform future development of our own dengue vaccine candidate, V181, which usesconfident that V116 represents a similar approach with the same antigenic content. In addition, we have begun a Phase 3 trial of our monoclonal antibody to provide passive immunity against respiratory syncytial virus in infants.multibillion-dollar opportunity.
Our Animal Health business remainedachieved solid growth in 2023, driven by balanced performance across both livestock and companion animal products. Our recently announced agreement to acquire the aqua business of Elanco Animal Health will, upon closing, establish Merck Animal Health as a market leader in 2022,this business segment. We are well positioned in Animal Health with consistent,a strong pipeline of livestock and companion animal products, and we expect to achieve above-market growth driven by higher demandover the long term.
Business development remains a priority and an integral element of our science-led business strategy at Merck, and in livestock from2023, we built on our ruminant, poultrystrong track record of identifying and swine products. Salesaccessing the best science to enhance our pipeline and drive long-term growth with several strategic acquisitions and new collaborations. We accelerated our presence in companion animal reflected higher demandimmunology with the acquisition of Prometheus Biosciences, and subsequently initiated a Phase 3 study for tulisokibart (MK-7240), a TNF-like ligand 1A (TL1A) antibody, in patients with ulcerative colitis. We announced a collaboration with Daiichi Sankyo for three potential first-in-class ADCs that provide the BRAVECTO parasiticide lineopportunity to develop meaningful new options for patients with certain types of products. We look forwardcancer and the opportunity to continuing to build our biopharmaceutical and technology offerings and driving growth, innovation and strong performance in Animal Health.
Our business development strategy is helping position Merck for growth well intodeliver the next decadegeneration of precision cancer medicines. We are also excited about our acquisition of Imago BioSciences, which expands our hematology presence. In addition, our 2024 acquisition of Harpoon Therapeutics augmented our oncology pipeline with a novel portfolio of T-cell engagers, including lead
candidate MK-6070, a T-cell engager targeting delta-like ligand 3 (DLL3), which is being evaluated in certain types of small-cell lung cancer and complements our diverse pipelineneuroendocrine tumors.
We remain deeply passionate about the work we do to enable access and product portfolio.have made strategic commitments to ensure a positive impact on global health. In 2022, we executed several important transactions, including the acquisition of Imago Biosciences and collaborations with Orion Corporation, Orna Therapeutics, Moderna Therapeutics and Kelun-Biotech. We will continue to take a disciplined and focused look at opportunities to add novel assets and technologies to our portfolio in 2023.
We are delivering on our longstanding commitment to operating responsibly, and in 2022, we continued to make significant progress across our goals of expanding access to health, developing a diverse and inclusive workforce, protecting our environment, and operating with the highest standards of ethics. We expanded access to our innovative portfolio to ensure Merck’s science advances global health. We are more than halfway toward reachingsurpassed our goal of providingto enable 100 million more people to access to our innovative portfolio globallyof medicines and vaccines – throughthree years ahead of schedule. As a result, we have raised our ambition and set a new goal to enable access for 350 million people. We have implemented several critical strategies solutionsto realize this goal, one of which focuses on collaboration with key financial institutions and partnerships,payers, helping them expand funding options that assist patients and their families with managing out-of-pocket medical costs due to critical illness. Additionally, guided by 2025. In addition, recognizing the ongoing need for multiple approachesour purpose and principles, we continue to treat COVID-19,uphold our long-term commitment of pricing our medicines responsibly.
Internally and across our global enterprise, we continued to accelerate global access to LAGEVRIO throughconsistently invest in our comprehensive supply and access approach.
We continued to cultivatecolleagues, foster a diversepositive and inclusive workforce, implementing critical strategiesworking environment and leveraging our 10 employee business resource groups, which represent the variousimprove representation across all dimensions of diversity that span our company. These groups enhance employee engagement, enable career growth and development, and help ensure we achieve our business objectives. We also made strong progress toward our climate goals and further developed the tools and processes required to reduce our company’s carbon footprint. And, we are committed to conducting ourselves with integrity, fully complying with regulatory requirements.
Our successdiversity. Importantly, in 2022 was underpinned bywomen represented over half of our understanding thatnew hires globally, and 47% of new hires in the workU.S. came from underrepresented ethnic groups. In the U.S., we do has a truly profound effect on global health. Throughout the year,also achieved greater than 99% pay equity for female and male employees, as well as non-white (including Black, Hispanic and Asian employees) and white employees.
In 2024, we continued to build on our legacy of putting patients first, focusing on how we can address unmet medical needs and work to prevent and treat devastating diseases. We will continue to leverage our size, scope and scale to grow and strength to invest inadvance our pipelinepipeline; and capitalize on opportunities that deliverto provide lifesaving and life-changing benefitsmedicines and vaccines to patients worldwide, as well as great returns forworldwide. Ultimately, this will enable us to deliver value to patients, employees, health care professionals, shareholders and all our shareholders. I am deeply inspired by and proud of the progress we’ve made. Still, I am keenly aware that our work is never done and that patients around the world are waiting for the kind of scientific innovation that Merck can uniquely deliver now and well into the future.
In 2022, we also marked the conclusion of a leadership transition as Ken Frazier retired from our company after a distinguished 30-year tenure. Throughout his Merck career, Ken set and exceeded a high bar of excellence in both executive and board leadership. He led with principle, embodied our core values, and made immense contributions to global health.stakeholders. On behalf of our entire company, I want tothe Company and the Board, thank Ken and wish him the best in his next chapter.
Thank you for your confidence andongoing support of our company. We hopeappreciate your partnership and perspectives and encourage you willto participate in the Annual Meeting either by attending virtually or by voting, as promptly as possible, through other alternative means as described in this proxy statement. Your participation is important, so please exerciseexercising your right to vote.
Robert M. Davis Chairman, Chief Executive Officer and President | “ In 2024, we will continue to leverage our size,
|
Merck & Co., Inc. 20232024 Proxy Statement
| 4 |
ç ç ç |
|
A Message from Merck’s Independent Lead Director
Dear Merck Shareholders,
As you read in the letter from our Chairman and CEO, Robert M. Davis, 2022shared in his letter, 2023 was a truly exceptionalvery strong year for the Company as it continued working to fulfill its purpose of using the power of leading-edge science to save and improve lives around the world. This purpose has guided the Company throughout its long history. It also guides my fellow Directors and methe Board in our work overseeing the Company’s affairs and fulfilling our responsibilities.
Both as a full Board and through our four standingindependent committees, composed of independent directors only, we are dedicated to the effective oversight of the Company’s strategy and business operations and the key risks the Companyit faces. Our annual self-evaluation process helps us identify ways to continue to enhance the overall effectivenessBoard leadership is an important component of the Board and its committees. It also gives us the opportunity to discuss other important topics through feedback from each of our Directors that is then considered by the full Board.
Part of our annual evaluation process includes aensuring effective oversight. We review of the Board’s leadership structure. Board leadership is a topic considered by the independent Directorsstructure at least annually to ensureconfirm the current structure remains the most appropriate leadership structure for the Company and the Board at a given time. In connection with the Company’s CEO transition in 2021, when the Board unanimously elected Mr. Davis to succeed Kenneth C. Frazier as CEO, and Mr. Frazier’s retirement as Executive Chairman at the end of 2022, in particular, the independent Directors gave significant consideration to the Board’s leadership structure. As independent Lead Director during this period, I led a process with my fellow Directors, working closely with Mr. Davis and Mr. Frazier, that found Rob to be an innovative and strategic leader with a deep understanding of the Company and its industry and well-suited to serve as Chairman of the Board. Effective December 1, 2022, Mr. Davis was unanimously elected by the Board to serve as Chairman.
The Board believescontinues to believe that having Mr. Davis serve as Chairman and CEO provides strategic and operational expertise and perspective to the Chairman role because he can draw on his detailed institutional knowledge of the Company and his industry experience. At the same time, we have strong independent oversight through (a) the key duties and responsibilities I discharge as independent Lead Director and (b) our four independent Board committees chaired by independent Directors. Together, we ensure that Merck achieves the highest level of corporate governance, which includes having diverse perspectives in the boardroom and dialogue with shareholders.
We are deliberate in ensuringBoard composition is, of course, essential to effective oversight. My fellow Directors and I believe that we haveour twelve Director nominees possess broad expertise, skills, experience, and perspectives that will facilitate the right mix of perspectives, skillsstrong oversight and expertisestrategic direction required to addressgovern the Company’s currentbusiness and anticipated needs as opportunitiesstrengthen and challenges facingsupport senior management. To help demonstrate this, and in response to shareholder feedback, this proxy statement includes not only an individualized skills matrix showing the Company evolve. Our Directorskey skills that each Director represents, but also a matrix sharing demographic information for each Director. In fulfilling our board responsibilities, we draw on theirour unique experiences to provide guidance on corporate strategy and monitor its implementation in areas such as research and development, capital allocation, risk management, operating results, human capital management and global manufacturing. The Board also provides oversight
In addition, our annual self-evaluation process helps us identify ways to continue to enhance the overall effectiveness of the Company’s ESG strategyBoard and performance as a whole andour committees. It also gives us the opportunity to discuss other important topics through our committees based on their specific areas of competency. This year, our Compensation and Management Development Committee approved for 2023 a Company Scorecard that will, for the first time, include ESG metrics. Our Company Scorecard helps translate our strategic priorities into operational terms that enable tracking and measurementfeedback from each of our progress and performance against annual operating goals andDirectors that is then considered by the long-term strategic drivers of sustainable value creation. These key objectives include our research and development pipeline and, beginning in 2023, two key strategic ESG priorities of enabling access to the Company’s innovative portfolio to patients around the world and engagement and inclusion of the Company’s employees.full Board.
We appreciate your investment in Merck and your support for the Board. We remain committed to serving you and the patients around the world that depend on Merck’s life-saving work.
 |
Thomas H. Glocer Independent Lead Director April |
Merck & Co., Inc. 20232024 Proxy Statement
| ÷ ÷ ÷ ÷ | 5 |
Contents
| Proxy Summary | 6 | ||||||
| Corporate Governance | 11 | ||||||
| 11 | |||||||
| 12 | |||||||
| 13 | |||||||
| 14 | |||||||
| 17 | |||||||
| 18 | |||||||
Board Succession Planning, Criteria for Board Membership, and Director Nomination Process | |||||||
| 22 | |||||||
| 23 | |||||||
| 25 | |||||||
Access and Pricing Transparency for Our Medicines and Vaccines | 26 | ||||||
Political Contributions and Lobbying Expenditure Oversight and Disclosure | |||||||
| Stock Ownership Information | |||||||
| Proposal 1. Election of Directors | |||||||
| Director Compensation | |||||||
| Proposal 2. Non-Binding Advisory Vote to Approve the Compensation of Our Named Executive Officers | |||||||
| Compensation Discussion and Analysis | |||||||
Merck & Co., Inc. 20232024 Proxy Statement
6 | | | | | |
Proxy Summary | ||||||||
|
This summary highlights information contained elsewhere in this proxy statement and does not contain all of the information that you should consider. You should read the entire proxy statement carefully before voting.
Date and Time Tuesday, May 9:00 a.m. ET | Record Date
| |
Location Via Webcast at www.virtualshareholdermeeting.com/
| ||
Voting Matters | Page(s) | Board’s Recommendation | ||||
Proposal 1 Election of Directors | FOR each Nominee | |||||
Proposal 2 Non-binding Advisory Vote to Approve the Compensation of our Named Executive Officers (Say-on-Pay) | FOR | |||||
Proposal 3
| ||||||
Ratification of Appointment of Independent Registered Public Accounting Firm for | FOR | |||||
Shareholder Proposals |
|
| ||||
Proposals Shareholder Proposals | AGAINST | |||||
Business Highlights
|

| $ | in total R&D expenses in
|
| |||
Capital Returned & Dividend Increase
|
$ | Capital Returned to Shareholders
|
| |||

Total Shareholder Return(1)
Year-End 2022 | ||
| 1-Year | 3-Year | |
| 48.4% | 12.1% | |
| 5-Year | ||
19.3%
| ||
(1) Relative Total Shareholder Return, a component of our Performance Share Unit program that is described on page 56, is calculated on a different basis.
| ||
Year-End 2023 | ||
| 1-Year | 3-Year | |
| 1.0% | 15.4% | |
| 5-Year | ||
11.7%
| ||
(1) Relative Total Shareholder Return, a component of our Performance Share Unit program that is described on pages 56-57, is calculated on a different basis.
| ||
Merck & Co., Inc. 20232024 Proxy Statement
Proxy Summary | ÷ ÷ ÷ ÷ | 7 |
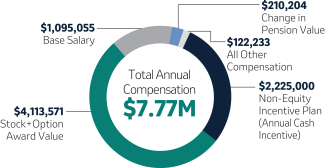
20222023 NEOs and Compensation Highlights (Page 48)50)
Below is a list of our 20222023 Named Executive Officers, or “NEOs”, and select compensation highlights from 2022.2023. For additional information on our elements of 20222023 compensation, please refer to the Compensation Discussion and Analysis (“CD&A”), beginning on page 43.45.
Annual Base Salary$
|
Target Annual Incentive%
|
Target Long-Term
|
Target TDC Increase%(1)
| |||||||||||||||||||||
|
2022 NEOs |
| ||||||||||||||||||||||
|
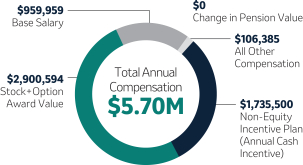
Robert M. Davis Chairman, Chief Executive Officer and President | $1,545,000 | 150% | $11,750,000 | +7.7% | |||||||||||||||||||
............................................................................................................................................................................. | ||||||||||||||||||||||||
 |
Kenneth C. Frazier Former Executive Chairman(5)
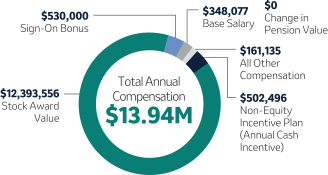
| 1,250,000 | 100(2) | 5,000,000 | -43.4 | |||||||||||||||||||
............................................................................................................................................................................. | ||||||||||||||||||||||||
 | Caroline Litchfield Executive Vice President and Chief Financial Officer
| 975,000 | 100 | 2,750,000 | +17.5 | |||||||||||||||||||
............................................................................................................................................................................. | ||||||||||||||||||||||||
 | Chirfi Guindo Chief Marketing Officer, Human Health
| 700,000 | 80 | —(3) | —(4) | |||||||||||||||||||
............................................................................................................................................................................. | ||||||||||||||||||||||||
 | Dean Li, M.D., Ph.D. Executive Vice President and President, Merck Research Laboratories
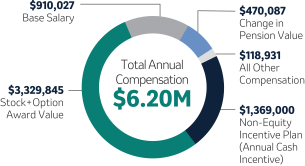
| 1,250,000 | 100 | 3,900,000 | +30.6 | |||||||||||||||||||
............................................................................................................................................................................. | ||||||||||||||||||||||||
 | Jennifer Zachary Executive Vice President and General Counsel
| 974,702 | 95 | 3,000,000 | +7.8 | |||||||||||||||||||
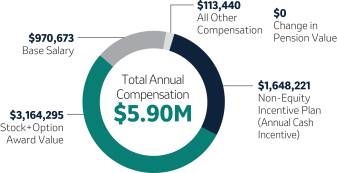
Annual Base Salary$(1)
|
|
Target Annual Incentive%
|
|
Target Long-Term
|
|
Target TDC Increase%(2)
| ||||||||||||||||||
|
2023 NEOs |
| ||||||||||||||||||||||
|
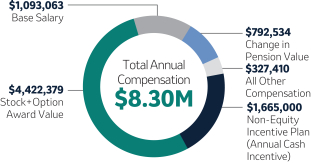
Robert M. Davis Chairman, Chief Executive Officer and President | $1,615,000 | 150% | $13,500,000 | +12.3% | |||||||||||||||||||
............................................................................................................................................................................. | ||||||||||||||||||||||||
 |
Caroline Litchfield Executive Vice President and Chief Financial Officer
| 1,125,000 | 100 | 4,250,000 | +38.3 | |||||||||||||||||||
............................................................................................................................................................................. | ||||||||||||||||||||||||
 | Sanat Chattopadhyay Executive Vice President and President, Merck Manufacturing Division
| 941,806 | 100 | 3,300,000 | —(3) | |||||||||||||||||||
............................................................................................................................................................................. | ||||||||||||||||||||||||
 | Richard R. DeLuca, Jr. Executive Vice President and President, Merck Animal Health
| 925,000 | 100 | 3,200,000 | +7.4(3) | |||||||||||||||||||
............................................................................................................................................................................. | ||||||||||||||||||||||||
 | Dean Li, M.D., Ph.D. Executive Vice President and President, Merck Research Laboratories
| 1,400,000 | 100 | 5,600,000 | +31.3 | |||||||||||||||||||
| (1) | Reflects base salary as of December 31, 2023. |
| (2) | Target Total Direct Compensation (“TDC”) is defined as the sum of annual base salary, target annual cash incentive and target long-term incentive. This column reflects the increase in Target TDC from |
| (3) | Mr. Chattopadhyay was not an NEO in |
|
|
|
|
Variable Compensation is Critical to Achieve Our Objectives (Page 51)53)
Merck’s compensation programs are designed to align the interests of our executives with the interests of our shareholders, among other objectives. For this reason, a significant portion of our NEOs’ pay is variable and at-risk, subject to Company performance as measured against financial, operating and strategic objectives, as well as Relative Total Shareholder Return or R-TSR (as defined in Appendix B). The Company’s variable incentives continue to demonstrate a strong linkage between pay and performance.
Annual Cash Incentive
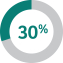
The Company Scorecard (described in more detail on page 53)55) focuses on our most critical business drivers — the Company’s revenue (“Revenue”), non-GAAP pre-tax income (“Pre-Tax Income”) and, the Company’s research and development goals (“Pipeline”), and the Company’s focus on driving greater access to health for patients around the incentive programworld and on the engagement and inclusion of our employees (“Pipeline”Sustainability”) — and is used to determine the payout of our annual incentive for all eligiblemost employees, including our NEOs under the Executive Incentive Plan. Our Company Scorecard performance during 20222023 resulted in above-target achievement of 178%148%.
Merck & Co., Inc. 20232024 Proxy Statement
| 8 |
ç ç ç |
Proxy Summary |
Long-Term Incentive (“LTI”)
The long-term incentive program, consisting of a mix of Performance Share Units (“PSUs”) and stock options, provides our NEOs with the opportunity to own Merck common stock, directly linking a substantial portion of their compensation to the returns realized by our shareholders.
The 20202021 PSU program (described in more detail on page 57)58) paid out at 110%161% based on achievement of one-year Earnings Per Share (“EPS”) and three-year R-TSR metrics during the performance period (2020-2022)(2021-2023), weighted at 33% and 67%, respectively. As previously disclosed, one-year EPS was used due to the complexities associated with disentangling our Organon & Co. (“Organon”) business from a multi-year financial plan. Organon was successfully spun off in June 2021.
| Say-On-Pay Advisory Vote (Page |
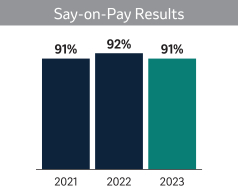 | |
| In |
We ask that our shareholders approve, on an advisory basis, the compensation of our NEOs as further described in Proposal 2 on page 42. This year, we are also asking shareholders to cast a non-binding, advisory vote on the frequency of future votes to approve the compensation of our NEOs. Shareholders will be able to specify one of four choices for this proposal on the proxy card; one year, two years, three years or abstain. The Board believes that it is appropriate and in the best interest for our shareholders to continue to cast a non-binding, advisory vote on the compensation of our NEOs on an annual basis.
44. For additional information, please refer to the CD&A beginning on page 4345 of this proxy statement.
Shareholder Engagement and Feedback (Page 25)24)
Merck communicates regularly with shareholders to better understand their perspectives and has established a shareholder engagement program that is both proactive and cross-functional. In addition, our independent Lead Director, who is also Chair of our Governance Committee, participates in substantive engagements with some of the Company’s largest shareholders. In 2022,2023, discussions with shareholders covered a wide range of topics of interest to shareholders, including Environmental, Social and Governance (“ESG”)sustainability reporting and goals, the Board’s leadership structure and composition, management and director succession, executive compensation programs, human capital management and other governance matters. These discussions provided valuable insights into shareholder views, and we heard from many shareholders that they greatly appreciated the opportunity to engage with our Company.
We will continue to engage with shareholders on a regular basis to better understand and consider their views, including on our ESGsustainability approach, our executive compensation programs and our corporate governance practices.
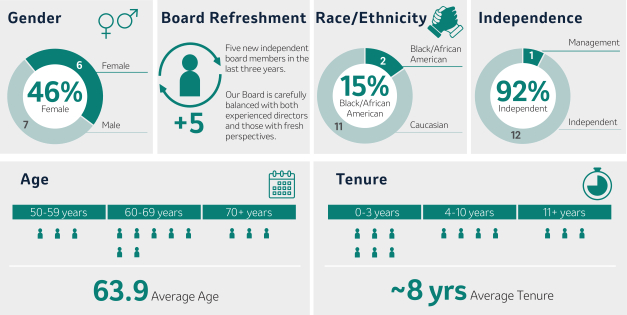
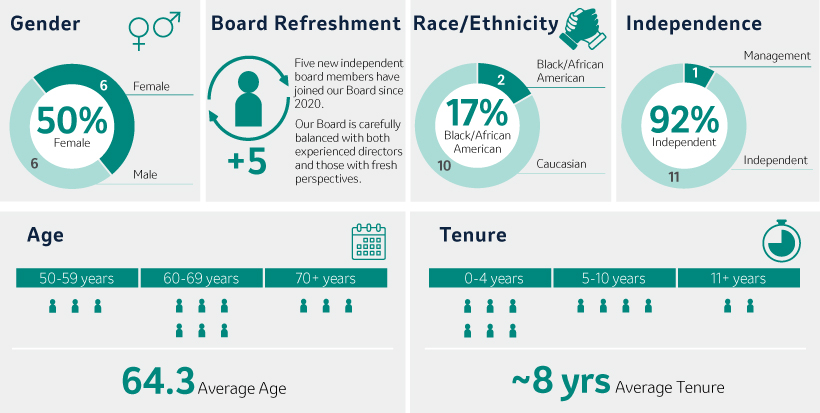
Board Composition and Refreshment
At least annually, the Governance Committee considers the size, structure and needs of the Board. The Governance Committee reviews possible candidates for the Board and recommends Director nominees to the Board for approval. In addition, as part of the Board’s annual self-evaluation process, Directors provide feedback on Board composition-related matters.
In selecting Director nominees, the Board considers its composition, including its diversity, and the skills, areas of expertise and experience then-represented on the Board. The Board also considers the Company’s current and future global business strategies, opportunities and challenges. Such considerations have resulted in the election of five new independent Directors over the last three years.since 2020. For more information, see “Board Succession Planning, Criteria for Board Membership, and Director Nomination Process” beginning on page 21.20. After years of dedicated service, Mr. Peter Wendell will retire from the Board effective as of the 2024 Annual Meeting.
Merck & Co., Inc. 20232024 Proxy Statement
Proxy Summary | ÷ ÷ ÷ ÷ | 9 |
Nominees for Director (Page 33)36)
The following provides summary information about each Director nominee. Each Director stands for election annually. Detailed information about each individual’s background, skillsets and areas of expertise can be found beginning on page 33.36.
| Current Committee Memberships | ||||||||||||||||||||||||||||||||||
| Audit | C&MD | Governance | Research | |||||||||||||||||||||||||||||||
| Director Nominee | Age | Director Since | Title |  |  |  |  | |||||||||||||||||||||||||||
| Douglas M. Baker, Jr. | 64 | 2022 | Former Executive Chairman and Chief Executive Officer, Ecolab Inc. |
|
| ||||||||||||||||||||||||||||
| Mary Ellen Coe | 56 | 2019 | Chief Business Officer, YouTube Inc. |
|
| ||||||||||||||||||||||||||||
| Pamela J. Craig | 66 | 2015 | Former Chief Financial Officer, Accenture plc |
|
| ||||||||||||||||||||||||||||
| Robert M. Davis Management | 56 | 2021 | Chairman, Chief Executive Officer and President, Merck & Co., Inc. | ||||||||||||||||||||||||||||||
| Thomas H. Glocer Lead Director | 63 | 2007 | Former Chief Executive Officer, Thomson Reuters Corporation |
|
| ||||||||||||||||||||||||||||
| Risa J. Lavizzo-Mourey, M.D. | 68 | 2020 | Professor Emerita, Robert Wood Johnson Foundation Population Health and Health Equity, University of Pennsylvania
|
|
| ||||||||||||||||||||||||||||
| Stephen L. Mayo, Ph.D. | 61 | 2021 | Bren Professor of Biology and Chemistry, California Institute of Technology |
|
| ||||||||||||||||||||||||||||
| Paul B. Rothman, M.D.
| 65 | 2015 | Former Dean of Medical Faculty and Vice President for Medicine, The Johns Hopkins University, and Former CEO, Johns Hopkins Medicine
|
|
| ||||||||||||||||||||||||||||
| Patricia F. Russo | 70 | 1995 | Chair, Hewlett Packard Enterprise Company; Former Chief Executive Officer and Director, Alcatel-Lucent
|
|
| ||||||||||||||||||||||||||||
| Christine E. Seidman, M.D. | 70 | 2020 | Thomas W. Smith Professor of Medicine and Genetics, Harvard Medical School, and Director, Cardiovascular Genetics Center, Brigham and Women’s Hospital
|
|
| ||||||||||||||||||||||||||||
| Inge G. Thulin | 69 | 2018 | Former Chairman of the Board, President and Chief Executive Officer, 3M Company
|
|
| ||||||||||||||||||||||||||||
| Kathy J. Warden | 51 | 2020 | Chair, Chief Executive Officer and President, Northrop Grumman Corporation |
|
| ||||||||||||||||||||||||||||
| Peter C. Wendell | 72 | 2003 | Managing Director, Sierra Ventures |
|
| ||||||||||||||||||||||||||||
Number of Meetings in 2022 | 9 | 5 | 4 | 5 | ||||||||||||||||||||||||||||||
| Current Committee Memberships | |||||||||||||||||||||||||||||||||||||
| Audit | C&MD | Governance | Research | ||||||||||||||||||||||||||||||||||
| Director Nominee | Age | Director Since | Title |  |  |  |  | ||||||||||||||||||||||||||||||
| Douglas M. Baker, Jr. | 65 | 2022 | Founding Partner, E2SG Partners; Former Executive Chairman and Chief Executive Officer, Ecolab Inc. |
|
| |||||||||||||||||||||||||||||||
| Mary Ellen Coe | 57 | 2019 | Chief Business Officer, YouTube Inc. |
|
| |||||||||||||||||||||||||||||||
| Pamela J. Craig | 67 | 2015 | Former Chief Financial Officer, Accenture plc |
|
| |||||||||||||||||||||||||||||||
| Robert M. Davis Management | 57 | 2021 | Chairman, Chief Executive Officer and President, Merck & Co., Inc. | |||||||||||||||||||||||||||||||||
| Thomas H. Glocer Lead Director | 64 | 2007 | Founder/Managing Partner, Angelic Ventures LP; Former Chief Executive Officer, Thomson Reuters Corporation |
|
| |||||||||||||||||||||||||||||||
| Risa J. Lavizzo-Mourey, M.D. | 69 | 2020 | Professor Emerita, Robert Wood Johnson Foundation Population Health and Health Equity, University of Pennsylvania
|
|
| |||||||||||||||||||||||||||||||
| Stephen L. Mayo, Ph.D. | 62 | 2021 | Bren Professor of Biology and Chemistry & Merkin Institute Professor, California Institute of Technology |
|
| |||||||||||||||||||||||||||||||
| Paul B. Rothman, M.D.
| 66 | 2015 | Former Dean of Medical Faculty and Vice President for Medicine, The Johns Hopkins University, and Former CEO, Johns Hopkins Medicine
|
|
| |||||||||||||||||||||||||||||||
| Patricia F. Russo | 71 | 1995 | Former Chief Executive Officer and Director, Alcatel-Lucent
|
|
| |||||||||||||||||||||||||||||||
| Christine E. Seidman, M.D. | 71 | 2020 | Thomas W. Smith Professor of Medicine and Genetics, Harvard Medical School, and Director, Cardiovascular Genetics Center, Brigham and Women’s Hospital
|
|
| |||||||||||||||||||||||||||||||
| Inge G. Thulin | 70 | 2018 | Former Chairman of the Board, President and Chief Executive Officer, 3M Company
|
|
| |||||||||||||||||||||||||||||||
| Kathy J. Warden | 52 | 2020 | Chair, Chief Executive Officer and President, Northrop Grumman Corporation |
|
| |||||||||||||||||||||||||||||||
Number of Meetings in 2023 | 9 | 4 | 4 | 4 | |||||||||||||||||||||||||||||||||
 Committee Chair
Committee Chair
Merck & Co., Inc. 20232024 Proxy Statement
| 10 |
ç ç ç |
Proxy Summary |
Our 20232024 Director Nominees Snapshot
Our Director nominees possess broad expertise, skills, experience and perspectives that will facilitate the strong oversight and strategic direction required to govern the Company’s business and strengthen and support senior management. As illustrated by the following charts, our slate of Director nominees consists of individuals with expertise in fields that align with the Company’s business and long-term strategy, includes a mixture of tenure that allows for both new perspectives and continuity and reflects the Board’s commitment to diverse perspectives.
Board Skills and Qualifications (of | No. of Nominees* | ||||
CEO Leadership | |||||
Financial | |||||
Scientific / Technology | |||||
| 6 | ||||
Healthcare Industry | 6 | ||||
Global Strategy & Operations | |||||
Marketing / Sales | 4 | ||||
Digital | |||||
Public Company Governance | |||||
Public Policy & Regulation | |||||
Talent Management | 10 | ||||
Capital Markets Experience | |||||
* See page 22 of this proxy statement under Individual Experience, Qualifications, AttributesNominee Skills and SkillsDemographic Matrix for how these Board skills and qualifications are represented by each Director nominee individually.
Merck & Co., Inc. 20232024 Proxy Statement
Corporate Governance |
| |||||||
| | | |
11
|
The Board has the legal responsibility for overseeing the affairs of the Company and for the overall performance of the Company. The Board’s primary mission is to represent and protect the interests of our shareholders. To that end, the Board selects and oversees the senior management team, which is charged with conducting Merck’s daily business.
The Board has adopted corporate governance guidelines (the “Policies of the Board”) that, together with our Restated Certificate of Incorporation, By-Laws and Board committee charters, form the governance framework for the Board and its committees. The Policies of the Board cover a wide range of subjects, including the philosophy and functions of the Board, the composition of the Board, the independent Lead Director’s responsibilities, categorical independence standards, Director qualifications, assessment of the Board, committee responsibilities, Director transition and retirement, service on other boards, Director compensation, stock ownership guidelines, chairmanship of meetings, Director orientation and continuing education, incumbent Director resignation and related person transactions. From time to time, the Board updates the Policies of the Board and Board committee charters in response to changing regulatory requirements, evolving best practices and the perspectives of our shareholders and other constituents.
Governance Materials
The following items relating to corporate governance at Merck are available on our website at www.merck.com/company-overview/leadership/board-of-directors:
| • | By-Laws |
Governance Highlights
We believe good corporate governance is essential to achieving long-term shareholder value. We are committed to governance policies and practices that serve the interests of our Company and its many stakeholders. For this reason, we devote considerable time and resources to making sure that our policies reflect our values and business goals, we have an effective corporate governance structure, and we operate in an open, honest and transparent way. In addition, we evaluate our practices against prevailing best practices as well as emerging and evolving topics identified in a variety of ways, including through shareholder engagement and corporate governance organizations.
Merck & Co., Inc.2023 2024 Proxy Statement
| 12 |
ç ç ç |
Corporate Governance Board Leadership Structure |
We highlight some significant aspects of our corporate governance practices below.
Independence
• We have a strong independent Lead Director.
• Our independent Directors convene regular executive sessions.
• All four of our standing Board committees (Audit, C&MD, Governance and Research) are comprised solely of independent Directors.
•
Accountability
• Every Director stands for re-election every year.
• Directors are elected by majority vote.
• An incumbent director who does not receive a majority vote must tender his/her resignation, and the Governance Committee must promptly make a recommendation as to the tendered resignation. The Board must act on the Governance Committee’s recommendation within 90 days after certification of the vote and publicly disclose its decision and rationale.
Best practices
• Our Board of Directors as a whole, and each Board committee, conducts a self-evaluation every year.
• The Board actively engages in CEO succession planning.
• The Board is diverse in terms of gender, ethnicity,
• Our Board has a written Diversity Policy incorporated into
Transparency
• We have strong control over our political spending and disclose corporate political activity and contributions in the U.S., Canada and Australia.
• We disclose aspects of our public policy engagement, including our key lobbying/advocacy issues.
• We disclose philanthropic grants and charitable contributions in the U.S. | Board oversight
• The full Board and each individual Board committee is responsible for overseeing risk.
• The full Board oversees corporate strategy.
Alignment with shareholder interests
• Our officers and directors are prohibited from engaging in hedging, pledging or short sale transactions involving
• Executives and Directors must hold prescribed meaningful amounts of
• We have a robust shareholder engagement program.
• We have a proxy access provision in our By-Laws under which shareholders who own 3% of our outstanding common stock for at least three years may nominate up to 20% of the members of our Board.
• Holders of 15% of our shares may call a special meeting.
• We do not have a shareholder rights plan (also known as a poison pill).
• We do not have any supermajority voting provisions.
Compensation practices
• We have conducted an annual say-on-pay advisory vote since 2011.
• All incentive compensation paid to executives is subject to a clawback policy.
• Our incentive compensation awards are designed to align pay with performance.
• Our C&MD Committee uses an independent compensation consultant.
Operating Responsibly
• We have a longstanding commitment to operating responsibly.
• All of our employees must adhere to a robust Code of Conduct. |
Board Leadership Structure
Currently, the Board is led by Robert M. Davis, who serves as the Chairman of the Board and CEO of Merck, and by Thomas H. Glocer, an independent Director, who serves as the Board’s Lead Director. The Board believes that the Company and our shareholders are best served by allowing the Board to exercise its judgment regarding the most appropriate leadership structure for the Company and the Board at a given time. The Board’s discretion should not be unduly constrained in advance because the most appropriate leadership structure at any given time will depend on a variety of factors, including the leadership, skills and experience of each of the CEO, the independent Lead Director and the other members of the Board, as well as the needs of the business and other factors.
Merck & Co., Inc. 2023 Proxy Statement
|
|
The independent Directors evaluate our Board leadership structure at least annually. In connection with the Company’s CEO transition in 2021, when the Board unanimously elected Mr. Davis to succeed Kenneth C. Frazier as CEO, and Mr. Frazier’s retirement as Executive Chairman at the end of 2022, in particular, the independent Directors gave significant consideration to the Board’s leadership structure. During this period, the independent Lead Director led a process with his fellow Directors working closely with Mr. Davis and Mr. Frazier that found Mr. Davis to be an innovative leader with deep understanding of the Company and its industry and well-suited to serve as Chairman of the Board. Effective December 1, 2022, Mr. Davis was unanimously elected by the Board to serve as the Chairman.
Merck & Co., Inc. 2024 Proxy Statement
Corporate Governance Lead Director | ÷ ÷ ÷ ÷ | 13 |
As Chairman, Mr. Davis presides over meetings of the Board and shareholders and focuses on Board operations and governance matters. He serves as the liaison between the Board and management, working closely with the independent Lead Director. Mr. Davis is also in charge of the general supervision, direction and control of the business and affairs of the Company subject to the Board’s overall oversight. The Board meets in executive session without Mr. Davis at each regular Board meeting. During these executive sessions led by Mr. Glocer as independent Lead Director, the Directors discuss topics such as the Board’s leadership structure, succession planning for the CEO and key management positions, and points of follow-up with management on strategic issues. The Board believes that having Mr. Davis serve as Chairman and CEO adds strategic and operational perspective to the Chairman role because he can draw on his detailed institutional knowledge of the Company and industry experience, while atexperience. At the same time, havingthere is strong independent oversight with Mr. Glocer as independent Lead Director vested with key duties and responsibilities and four independent Board committees chaired by independent Directors. Mr. Glocer can communicate with Mr. Davis between meetings and act as a “sounding board” and advisor and is also vested with key duties and responsibilities as discussed below.
Lead Director
Merck’s independent Lead Director is appointedAppointed by the independent members of the Board of Directors, to a three-year term. Thethe position of Lead Director has a clear mandate and significant authority and responsibilities set forth in the Policies of the Board, including:Board. These include:
Board Meetings and Executive Sessions | • The authority to call meetings of the independent members of the Board.
• Presiding at all meetings of the Board at which the Chairman is not present, including executive sessions of the independent members of the Board. | |
Communicating with
| • Serving as the principal liaison on Board-wide issues between the independent members of the Board and the Chairman and CEO. | |
Agendas | • Approving meeting agendas and information sent to the Board, including supporting material for meetings. | |
Meeting Schedules | • Approving meeting schedules to ensure there is sufficient time for discussion of all agenda items. | |
Communicating with Shareholders and Stakeholders | • Being available for consultation and direct communication with major shareholders, as appropriate.
• Serving as a liaison between the Board and shareholders on investor matters. | |
Board Performance Evaluation | • Leading the annual performance evaluation of the Board. | |
Chairman and CEO Performance Evaluations | • Leading the annual performance evaluation of the Chairman and CEO. | |
CEO Succession | • Leading the CEO succession planning process. | |
Merck & Co., Inc. 2023 Proxy Statement
|
|
As further described below,Each of the Board’s four standing committees each of which is composed solely of independent Directors,Directors. As further described below, each of these committees also playplays an active role in the Board’s leadership structure. The independent chairs of each of these committees provide strong leadership to guide the important work of the Board. They work with the Company’s senior executives to ensure the committees are discussing key strategic risks and opportunities of the Company. The Board believes the Company and its shareholders are well-served by the current leadership structure for all the foregoing reasons.
Merck & Co., Inc. 2024 Proxy Statement
| 14 | ç ç ç ç | Corporate Governance Board Meetings and Committees |
Board Meetings and Committees
In
The independent Directors of the Board met in |
All Directors attended at least 75% of the meetings of the Board and of the committees on which they served in |
The Board of Directors has four standing committees, each of which is made up solely of independent Directors: Audit Committee; C&MD Committee; Governance Committee; and Research Committee. In addition, the Board from time to time establishes special purpose committees. All of ourthe standing committees are governed by Board-approved charters, which are available on our website at www.merck.com/company-overview/leadership/board-of-directors/. The committees evaluate their performance and review their charters annually. Additional information about the committees is provided below. As a non-independent director, Mr. Davis is not a member of any Board committee, but may participate in meetings at the request of the committees.
Merck & Co., Inc. 20232024 Proxy Statement
Corporate Governance Board Meetings and Committees | ÷ ÷ ÷ ÷ | 15 |
Audit Committee
|
|
| Pamela J. Craig Chair | Overview
The Audit Committee oversees our accounting and financial reporting processes, internal controls and audits and consults with management, the internal auditors, and the independent auditors on, among other items, matters related to the annual audit, the published financial statements and the accounting principles applied. The Audit Committee has established policies and procedures for the pre-approval of all services provided by the independent auditors (as described on page
The Audit Committee’s Report is included on page
The Primary Functions of this Committee are to:
• Oversee the Company’s accounting and financial reporting processes, internal controls and audits;
• Appoint, evaluate and retain, and maintain direct responsibility for the compensation, termination and oversight of, the Company’s independent auditors, including evaluating their qualification, performance, and independence;
• Oversee the Company’s compliance with legal & regulatory requirements, including monitoring compliance with the Foreign Corrupt Practices Act and the Company’s policies on ethical business practices and reporting on these items to the Board;
• Establish procedures for the receipt, retention and treatment, on a confidential basis, of
• Oversee the Enterprise Risk Management process;
• Regularly meet with the Chief Information Officer regarding the Company’s information technology and have primary responsibility for overseeing the Company’s cybersecurity risk management program; and
• Review any significant issues concerning litigation and contingencies with management, counsel, and the independent public accountants. | |||||||
|
Other Members Douglas M. Baker, Jr. Stephen L. Mayo, Ph.D. Paul B. Rothman, M.D. Christine E. Seidman, M.D. Kathy J. Warden
| ||||||||
Number of Meetings in 9
| |||||||||
Financial Experts on Audit Committee
The Board has determined that each of Mr. Baker, Ms. Craig and Ms. Warden is an “audit committee financial expert” as defined by the SEC and has accounting or related financial management expertise as required by NYSE Corporate Governance Listing Standards.
| |||||||||
(1) Mr. Baker was appointed to the Audit Committee as of March 27, 2023. He previously served on the C&MD Committee.
Compensation and Management Development Committee
|
|
| Patricia F. Russo Chair | Overview
The C&MD Committee annually reviews and approves corporate goals and objectives relevant to the compensation opportunity for the Company’s executive officers, including the
The C&MD Committee Report is included on page
The Primary Functions of this Committee are to:
• Establish and maintain a competitive portfolio of fair and equitable compensation and benefits policies, practices and programs designed to attract, engage and retain a workforce that helps the Company achieve immediate and long-term success;
• Discharge the Board’s responsibilities for compensating our officers;
• Oversee/monitor – The competence and qualifications of our executive officers, – Officer succession, – The soundness of the organizational structure, – The Company’s programs, policies and practices related to its management of human – Other related matters necessary to ensure the effective management of the business; and
• Review the Compensation Discussion and Analysis for inclusion in our proxy statement. | |||||||
|
Other Members Mary Ellen Coe Thomas H. Glocer Risa J. Lavizzo-Mourey, M.D. Inge G. Thulin Peter C.
| ||||||||
Number of Meetings in
| |||||||||
Compensation and Management Development Committee Interlocks and Insider Participation
There were no C&MD Committee interlocks or insider (employee) participation during
| |||||||||
(1)Ms. Coe was appointed to Mr. Wendell is retiring from the C&MD CommitteeBoard effective as of March 27, 2023. She previously served on the Audit Committee.2024 Annual Meeting.
Merck & Co., Inc. 20232024 Proxy Statement
| 16 |
ç ç ç |
Corporate Governance Board Meetings and Committees |
Governance Committee
|
|
| Thomas H. Glocer Chair | Lead Director
| Overview
The Governance Committee oversees the Company’s corporate governance, including the practices, policies and procedures of the Board and its committees. Further, the Governance Committee annually reviews the size, structure and needs of the Board and Board committees, reviews possible candidates for the Board and recommends Director nominees to the Board for approval. The details of the review process and assessment of candidates are described under “Board Succession Planning, Criteria for Board Membership, and Director Nomination Process” beginning on page
The Primary Functions of this Committee are to:
• Coordinate an annual evaluation of Board performance, and review Board compensation, related person transactions and
• Oversee the Board’s Incumbent Director Resignation Policy;
• Review the Company’s Good Manufacturing Practice compliance, including with respect to internal and external manufacturing as well as internal and external audits; worker safety practices; and privacy policies and practices;
• Review social, political and economic trends that affect our business; review the positions and strategies we pursue to influence public policy; and
• Assist the Board in its oversight of the Company’s | |||||||
|
Other Members Douglas M. Baker, Jr. Pamela J. Craig Patricia F. Russo Inge G. Thulin Kathy J. Warden
| ||||||||
Number of Meetings in 4
| |||||||||
| |||||||||
Research Committee
|
|
| Paul B. Rothman, M.D. Chair | Overview
The Research Committee oversees the overall strategy, direction and effectiveness of the Company’s operations for the research and development of pharmaceutical products and vaccines. As part of this oversight, the Research Committee focuses on a variety of areas, including drug and vaccine discovery, licensing and development strategies, decision-making procedures and outcomes, as well as processes and procedures for identifying, evaluating and capitalizing on cutting edge scientific developments and advancements and enabling technologies.
The Primary Functions of this Committee are to:
• Identify areas and activities that are critical to the success of our product and vaccine discovery, development and licensing efforts and evaluate the effectiveness of our strategies and operations in those areas;
• Keep the Board apprised of this evaluation process and findings and make appropriate recommendations to the President of Merck Research Laboratories and to the Board on modifications of strategies and operations; and
• Assist the Board in its oversight responsibilities to ensure compliance with the highest standards of scientific integrity in the conduct of Merck research and development. | |||||||
|
Other Members Mary Ellen Coe Risa J. Lavizzo-Mourey, M.D. Stephen L. Mayo, Ph.D. Christine E. Seidman, M.D. Peter C. Wendell(1)
| ||||||||
Number of Meetings in
| |||||||||
(1) Mr. Wendell is retiring from the Board effective as of the 2024 Annual Meeting.
Merck & Co., Inc. 20232024 Proxy Statement
Corporate Governance Board’s Role in Strategic Planning | ÷ ÷ ÷ ÷ | 17 |
Board’s Role in Strategic Planning
The Board — acting both as a whole and through its four standing committees — is fully engaged and involved in the Company’s strategic planning process. All of our Directors have an obligation to keep informed about the Company’s business and strategies, so they can provide guidance to management in formulating and developing plans and knowledgeably exercise their decision-making authority on matters of importance to the Company.
The Board’s oversight and guidance are inextricably linked to the development and review of the Company’s strategic plan. By exercising sound and independent business judgment on the strategic issues that are important to the Company’s business, the Board facilitates Merck’s long-term success.
Our Strategic Planning Cycle


Merck & Co., Inc. 20232024 Proxy Statement
| 18 |
ç ç ç |
Corporate Governance Risk Oversight |
Risk Oversight
Overseeing risk is an important component of the Board’s engagement on strategic planning. The Board’s approach to overseeing risk management leverages the Board’s leadership structure and ensures the Board oversees risk through both a Company-wide approach and specific areas of competency. A summary of this risk oversight approach follows:
Board of Directors
Oversees risk through Company-wide Enterprise Risk Management (“ERM”) process and functioning of Board Committees. |
Audit Committee
Responsibility for reviewing the ERM process to ensure it is robust and functioning effectively.
Primary responsibility for overseeing the Company’s cybersecurity risk management
Oversees risk relating to finance, business integrity and internal controls over financial reporting through its interactions with the Chief Financial Officer, Chief Compliance Officer, Controller and the head of internal audit.
| |||
Compensation and Management Development Committee
Evaluates relationships between risk and reward as it relates to our executive compensation program.
When setting incentive plan targets each year, the C&MD Committee is aware of the risk associated with drug pricing, among other things, and ensures our plans do not incentivize risky behavior in order to meet targets.
Oversees the Company’s programs, policies and practices related to its management of human capital resources.
| ||||
Management
Identification, assessment and management of risk through Company-wide ERM process. | ||||
Governance Committee
Oversees the Company’s corporate governance, including the practices, policies and procedures of the Board and its committees, considers the size, structure and needs of the Board, reviews possible candidates for the Board, and recommends Director nominees to the Board for approval.
Plays a role in compliance oversight, including in the areas of manufacturing quality, privacy, and worker safety.
Assists the Board in its | ||||
Research Committee
Oversees overall strategy, direction and effectiveness of the Company’s research and development operations.
|
The ERM process allows for full Board oversight of the most significant risks facing the Company and was established to ensure a complete Company-wide approach to evaluating risk over six distinct but overlapping risk areas:
Responsibility and Reputation | Risks that may impact the well-being of the Company, its employees, customers, patients, communities or reputation | ||
Strategy | Macro risks that may impact our ability to achieve long-term business objectives | ||
Operations | Risks in operations and cybersecurity that may impact our ability to achieve business objectives | ||
Compliance | Risks related to compliance with laws, regulations and Company values, ethics and policies | ||
Reporting | Risks to maintaining accurate financial statements and timely, complete financial disclosures | ||
Safety | Risks to employee, patient or community health and safety | ||
Merck & Co., Inc. 20232024 Proxy Statement
Corporate Governance Risk Oversight | ÷ ÷ ÷ ÷ | 19 |
Our ERM process seeks to identify emerging risks and address them appropriately to limit negative consequences to the Company and the data it maintains. Its goal is to provide an ongoing review, implemented across the Company and aligned to Company values and ethics, to identify and assess risk and to monitor risk and agreed-upon mitigating action. Furthermore, if a risk transforms into an incident, the ERM process ensures that effective response and business continuity plans are in place. If the ERM process identifies a material risk, it will be elevated through the CEO and the Executive Team to the full Board for consideration. Through the ERM process, each Board committee oversees specific areas of risk relevant to the committee through direct interactions with the CEO, members of the Company’s Executive Team and the heads of business divisions, compliance and corporate functions. A committee may address risks directly with management or, where appropriate, may elevate a risk for consideration by the full Board or another Board committee. The Board committees also oversee risk based on their specific areas of competency. Additional detail with respect to certain key areas of oversight are provided below.
Cybersecurity and Privacy
As our Company becomesdiscussed in more dependentdetail in the 2023 10-K, the Company’s cybersecurity measures are primarily focused on ensuring the security and protection of its information technology systems and data,data. The Audit Committee and the need for a robustBoard receive periodic reports that include updates on the Company’s cybersecurity privacy,risks and technology riskthreats, the status of projects intended to strengthen its information security systems, assessments of the information security program (including remediation, mitigation, and management of identified vulnerabilities), and the emerging threat landscape. The Company’s information security program is increasingly critical. We have developedregularly evaluated by internal and implemented a comprehensive program designedexternal consultants and auditors with the results of those reviews reported to protect the confidentiality of sensitive information, ensure the integrity of critical data and automated processes, and safeguard the availability of our information technology capabilities.
Cybersecurity has been an area ofsenior management attention for over two decades and we have aligned our cybersecurity program to the National Institute of Standards and Technology (NIST) Cybersecurity Framework and the Payment Card Industry Data Security Standard (PCI-DSS). We have implemented appropriate policies, processes, and technology to reduce the likelihood or impact of a breach and have cyber insurance. We have an employee awareness program to regularly educate our workforce on the cybersecurity risks they face and how they can operate safely. We regularly assess our cybersecurity capabilities using third party security firms.Audit Committee.
We have also developed and continually evolve our Global Privacy Program to promote organizational accountability for privacy, data governance, and data protection across our business and with our collaborative partners and suppliers. The program helps us uphold our commitment to data security and privacy, including maintaining 100% compliance to regulatory requirements for active incident monitoring, risk/harm analysis, and on-time notification of data breaches. Our commitment applies not only to our Company’s information, but also to the information entrusted to us by others. We were the first company in the world to obtain regulatory certification in the European Union for Binding Corporate Rules based in part on our existing Asia Pacific Economic Cooperation Cross Border Privacy Rules certification.
We are aware that we must continuously evolve our controls to address new threats, adhere to changing laws and standards, and reduce the risk associated with the introduction of new, innovative technology. While everyone at the Company plays a part in information security, cybersecurity, and data privacy, oversight responsibility is shared by the Board, its committees, and management.
Responsible Party | Oversight Area for Cybersecurity and Privacy Matters | |
Board | Participates in regular reviews and discussions dedicated to the Company’s risks related to the protection of our data and systems including cybersecurity and privacy. | |
Audit Committee |
| |
Governance | Responsible for oversight in the area of privacy and receives periodic updates regarding the Company’s Global Privacy Program. | |
Management | Responsible for implementing and managing the Company’s framework for assessing, prioritizing and mitigating cybersecurity risk. A Chief Information Security Officer manages the Company’s Information Security Program with a group responsible for leading enterprise-wide cybersecurity risk management, strategy, policy, standards, architecture and processes. Manages the Company’s Global Privacy Program. Responds to incidents and issues in a timely manner. Provides periodic updates to the Board and or its committees, as applicable. |
Merck & Co., Inc. 20232024 Proxy Statement
| 20 |
ç ç ç |
Corporate Governance
|
Environmental, Social and Governance (“ESG”) MattersSustainability
The Board of Directors and its Committees are responsible and accountable for overseeing the Company’s ESGsustainability matters, whileand management is responsible for reviewing, refining, and implementing the long-term ESGsustainability strategy and for updating the Board and its Committees.
Responsible Party | Key Oversight | |
Board | The | |
Governance | This Committee monitors and assists the Board in | |
C&MD Committee | This Committee assists the Board with its oversight of human capital management, including the Company’s programs, policies, and practices related to talent management, culture, diversity, equity and inclusion. This includes maintaining fair hiring and promotion practices, and a commitment to sustain equitable pay | |
Audit Committee | This Committee monitors compliance with the Company’s policies on ethical business practices and oversees any sustainability bonds the Company may issue. | |
Research Committee | This Committee monitors compliance with the highest standards of scientific integrity in the conduct of the Company’s research and development. | |
Management | Our Executive Team and senior management are responsible for reviewing, refining and implementing our Company’s long-term
By ensuring ongoing business engagement ownership and accountability with regard to |
For more information about our approach to ESG,sustainability, please visit our 2021/2022 ESG Progress2022/2023 Impact Report at https://www.merck.com/company-overview/esg/sustainability/.
Providing Access to Health to Patients Around the World
We are continuing to make progress in ESG areas that matter most to our Company and our stakeholders which include Access to Health, Employees, Environmental Sustainability, and Ethics & Values. Of these four priorities, Access to Health is central to our purpose as a research-intensive biopharmaceutical company, helping to create value for our stakeholders and improve the lives of patients around the world. Our approach is guided by our Access to Health Guiding Principles, focused in four main areas:
Discovery and invention
Availability
Affordability
Strengthening health care systems and addressing inequity
Merck & Co., Inc. 2023 Proxy Statement
|
|
Our Access to Health Guiding Principles (the “Principles”) guide our work in addressing global burdens of disease with our products and pipeline. We aim to make a safe global supply of quality medicines and vaccines available to the greatest number of patients today while meeting the needs of patients in the future. The Principles inform our efforts to address barriers to accessing our medicines and vaccines, as well as the systemic barriers to health care around the world.
Discovery and Invention
Pursuing the most promising science, we discover and invent medicines and vaccines that address vital global health needs where we can have the greatest impact, now and in the future.
Availability
Making available a reliable and safe global supply of quality medicines and vaccines, we invest in solutions to enable timely access to our products, in a responsible and sustainable manner.
Affordability
Grounded in a deliberate systematic approach, we develop, test, and implement innovative solutions that address barriers to access and affordability of our medicines and vaccines.
Strengthening Systems and Addressing Inequity
Applying our expertise to address systemic barriers to access to health, we believe we can make the strongest contributions to health systems, communities and our patients around the world.
Guided by these Principles, we will continue to expand access to our products through market-based solutions that help address unmet need, taking into consideration public health need, economic conditions and health-care infrastructure.
Board Succession Planning, Criteria for Board Membership, and Director Nomination Process
In Board succession planning, the Governance Committee and the Board consider, among other things, the needs of the Board and the Company in light of the overall composition of the Board, with a view toward achieving a balance of the skills, experience and attributes that are essential to the Board’s oversight role. In particular, the Board is deliberate in ensuring the Board has the right mix of diverse perspectives, skills and expertise to address the Company’s current and anticipated needs as opportunities and challenges facing the Company evolve. The Board also strives to ensure an appropriate mix of tenure on the Board and being balanced with both experienced and newer directors. Such considerations have resulted in the election of five new independent Board members over the last three years.since 2020. The Board also has a policy set forth in the Policies of the Board that Directors may not be nominated for re-election to the Board after they reach the age of 75. The Board believes that, in addition to its ongoing review of the overall composition of the Board, this policy promotes regular refreshment of the Board and is considered as part of overall succession planning.
Merck & Co., Inc. 2024 Proxy Statement
Corporate Governance Board Succession Planning, Criteria for Board Membership, and Director Nomination Process | ÷ ÷ ÷ ÷ | 21 |
The Governance Committee is responsible for screening and nominating director candidates to be considered for election by the Board. As part of this process, the Governance Committee considers the composition of the Board at the time, including the depth of experience, balance of professional skills, expertise and diversity of perspectives represented by its members at the time. The Governance Committee evaluates prospective nominees identified on its own initiative as well as candidates recommended by other Board members, management, shareholders or search consultants. In 2022, the Governance Committee retained a search firm to identify possible candidates who meet the Board’s qualifications, to interview and screen such candidates (including conducting reference checks) and to assist in scheduling candidate interviews with Board members.
To be considered for membership on the Board, a candidate must meet the following minimum criteria:
be of proven integrity with a record of substantial achievement in an area of relevance to the Company;
have demonstrated ability and sound judgment that usually will be based on broad experience;
be able and willing to devote the required amount of time to the Company’s affairs, including attendance at Board meetings, Board committee meetings and annual shareholder meetings;
possess a judicious and critical temperament that will enable objective appraisal of management’s plans and programs; and
be committed to building sound, long-term Company growth.
Merck & Co., Inc. 2023 Proxy Statement
|
|
Individual Experience, Qualifications, Attributes and Skills
In its regular discussions regarding Board composition — and especially in conjunction with the annual Board and committee evaluations — the Governance Committee works with the Board to determine the appropriate mix of professional experience, expertise, educational background and other qualifications that are particularly desirable in light of our current and future business strategies. The Governance Committee uses this input in its planning and Director search process. In addition to the five broad criteria listed above, the following chart highlights the key skills and qualifications the Board considers for future candidates. As reflected below, these attributes are amply represented by our current Director nominees.
Board Skills and Qualifications
 |  |  |  |  |  |  |  |  |  |  |  |  | ||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
Board Skills and Qualifications Categories
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
Merck & Co., Inc. 2023 Proxy Statement
|
|
Diversity
As a Company, Merck knows that diversity and inclusion are fundamental to the Company’s success and core to future innovation. As a Board, diversity is an important factor considered when identifying prospective nominees for our Board, and the Policies of the Board include a formal diversity policy. The policy reflects the Board’s longstanding commitment to ensuring that Directors represent diverse perspectives and areas of expertise important to fostering the Company’s business success. The policy provides that the Board does not discriminate against potential Directors on the basis of gender, race, age, sexual orientation or ethnic and national background and that having a board composed of diverse individuals is an important contributor to the Board’s overall effectiveness.
Shareholder Recommendations of Director Candidates
The Governance Committee will consider recommendations for Director candidates made by shareholders and will evaluate those individuals using the same criteria applied to other candidates. Shareholder recommendations must be sent to the Office of the Secretary, Merck & Co., Inc., 126 East Lincoln Avenue, Rahway, N.J. 07065 U.S.A., and must include detailed background information regarding the recommended candidate that demonstrates how that candidate meets the Board membership criteria.
Candidates are evaluated initially based on materials submitted by them or on their behalf. If a proposed or recommended candidate continues to be of interest to the Governance Committee, we obtain additional information through inquiries to various sources and, if warranted, interviews.
Individual Experience, Qualifications, Attributes and Skills
In its regular discussions regarding Board composition — and especially in conjunction with the annual Board and committee evaluations — the Governance Committee works with the Board to determine the appropriate mix of professional experience, expertise, educational background and other qualifications that are particularly desirable in light of our current and future business strategies. The Governance Committee uses this input in its planning and Director search process.
To be considered for membership on the Board, a candidate must meet the following minimum criteria:
be of proven integrity with a record of substantial achievement in an area of relevance to the Company;
have demonstrated ability and sound judgment that usually will be based on broad experience;
be able and willing to devote the required amount of time to the Company’s affairs, including attendance at Board meetings, Board committee meetings and annual shareholder meetings;
possess a judicious and critical temperament that will enable objective appraisal of management’s plans and programs; and
be committed to building sound, long-term Company growth.
In addition to these criteria, the following matrix highlights the mix of key skills and qualifications the Board considers for future candidates. These attributes are amply represented by our current Director nominees. Additional biographical information on each nominee is set out starting on page 36.
Merck & Co., Inc. 2024 Proxy Statement
| 22 | ç ç ç ç | Corporate Governance Nominee Skills and Demographic Matrix |
The following biographical disclosures include information regarding nominees for election at the 2024 Annual Meeting only. After years of dedicated service, Peter Wendell will retire as of, and not stand for reelection at, the 2024 Annual Meeting, and is not represented in the following biographical disclosures.
Nominee Skills and Demographic Matrix
Skills and Experience of our 2024 Director Nominees |  |  |  |  |  |  |  |  |  |  |  |  | ||||||||||||
CEO Leadership Experience serving as a chief executive officer at a publicly traded or private organization | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||||||||
Financial Experience or expertise in financial accounting and reporting processes or the financial management of a major organization | ● | ● | ● | ● | ● | ● | ||||||||||||||||||
Scientific / Technology Scientific or technological degree or work experience in a scientific or technological field | ● | ● | ● | ● | ● | ● | ||||||||||||||||||
Healthcare Industry Experience with complex issues within the healthcare industry | ● | ● | ● | ● | ● | ● | ||||||||||||||||||
Global Strategy & Operations Leadership experience overseeing and/or driving strategic direction and growth of an organization globally | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||||||
Marketing / Sales Experience in marketing, advertising, social media and consumer insight functions, including product development and brand building | ● | ● | ● | ● | ||||||||||||||||||||
Digital Experience or expertise in information technology (including cybersecurity and data privacy) or the use of digital media to facilitate business objectives | ● | ● | ● | ● | ● | ● | ● | |||||||||||||||||
Public Company Governance Experience as a board member of another publicly-traded company | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||||||
Public Policy & Regulation Experience with public policy and regulation in the healthcare industry or other highly- regulated industries | ● | ● | ● | ● | ● | ● | ● | |||||||||||||||||
Talent Management Experience in executive recruiting, succession planning and talent management, including retaining key talent and driving employee engagement | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||||||
Capital Markets Experience Experience in corporate lending or borrowing, capital market transactions, significant mergers or acquisitions, private equity or investment banking | ● | ● | ● | |||||||||||||||||||||
Certain Demographic Information | ||||||||||||||||||||||||
Tenure (years) | 2 | 5 | 9 | 3 | 17 | 4 | 3 | 9 | 29 | 4 | 6 | 4 | ||||||||||||
Age | 65 | 57 | 67 | 57 | 64 | 69 | 62 | 66 | 71 | 71 | 70 | 52 | ||||||||||||
Gender | M | F | F | M | M | F | M | M | F | F | M | F | ||||||||||||
Race/Ethnicity | ||||||||||||||||||||||||
Black/African American | ● | ● | ||||||||||||||||||||||
White | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||||||||||
Merck & Co., Inc. 2024 Proxy Statement
Corporate Governance Management Succession Planning | ÷ ÷ ÷ ÷ | 23 |
Management Succession Planning
Succession planning and talent development are important at all levels within the Company. The Board regularly reviews short- and long-term succession plans for the CEO and other executive officers. In assessing possible CEO candidates, the independent Directors identify the skills, experience and attributes they believe are required for an effective CEO in light of the Company’s global business strategies, opportunities and challenges. More broadly, the Board engages with the Company’s leadership team on matters of talent and culture, including around the development of the Company’s talent pipeline and advancing diversity and inclusion efforts across the enterprise. The Board’s succession planning activities are strategic, long-term and supported by the Board’s committees and external consultants, as needed, and Directors have substantial opportunities to engage with possible succession candidates.
Merck & Co., Inc. 2023 Proxy Statement
|
|
Annual Board Evaluation
The Board conducts an evaluation of its performance and effectiveness, as well as that of its four standing committees, on an annual basis. The purpose of the evaluation is to track progress in certain areas targeted for improvement, identify ways to enhance the overall effectiveness of the Board and its committees, track progress in certain areas targeted for improvement, and provide opportunities to discuss other important topics. Among other topics, each Director provides feedback on the following topics that the full Board then considers:
the Board’s effectiveness in evaluating and monitoring the Company’s strategy and risks;
whether the Board has the right mix of skills, experiences, and attributes in light of the current issues facing the Company and its strategy for the future;
whether the Board’s leadership structure remains appropriate and effective;
evaluating such Director’s contributions as well as the contributions of other Directors; and
whether there is adequate time to raise questions, and make comments, both during and between Board meetings.
In addition, each Committee member evaluates their respective Committees, including with respect to leadership, composition, and functioning. The independent Lead Director leads the evaluation process. In 2022,2023, the evaluation was conducted in 3 phases.phases, and led to refinements to meeting agendas, streamlining of materials, and identification of priority agenda topics for the Board to address in 2024.
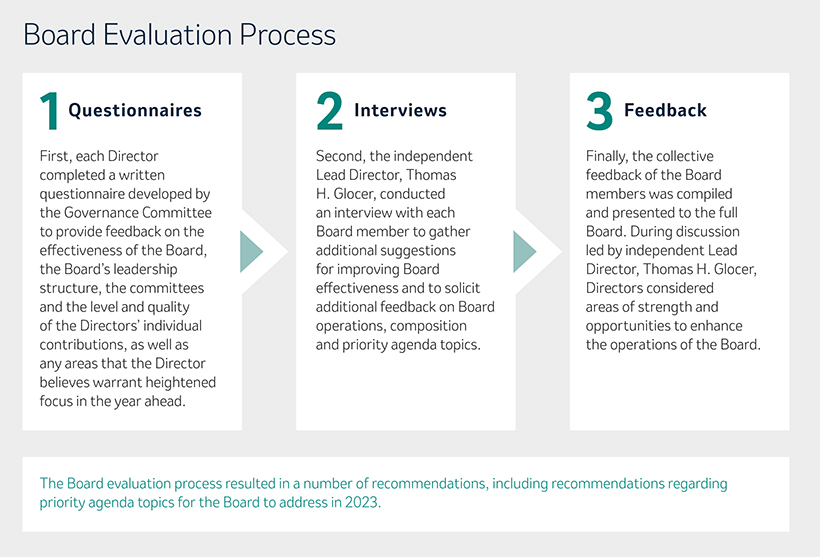
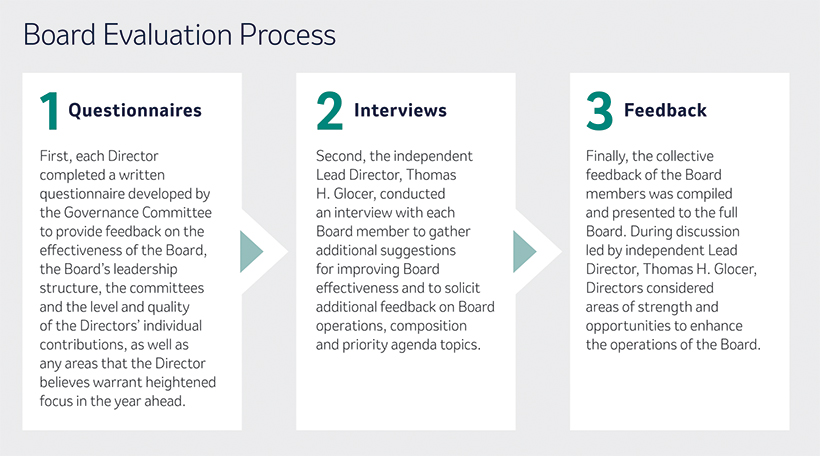
Merck & Co., Inc. 20232024 Proxy Statement
| 24 | ç ç ç ç |
Corporate Governance Shareholder Engagement and Feedback |
Shareholder Engagement and Feedback
Merck regularly communicates with shareholders to better understand their perspectives and has established a shareholder engagement program that is proactive and cross-functional. Throughout the year, members of Investor Relations, the Office of the Secretary, Human Resources and the ESG Strategy and Engagement Team, as well as other subject-matter experts within the Company, engage with our shareholders to remain well-informed regarding their perspectives on current issues and to address any questions or concerns. These teams serve as liaisons between shareholders, members of senior management and the Board. We also regularly seek to take advantage of other engagement opportunities and events.
In addition, we conduct a formal shareholder outreach program twice a year focused on governance, executive compensation and ESG matters.sustainability. We believe it is most productive to discuss these matters well in advance of the Company’s Annual Meeting of Shareholders to enable management and the Board to gather information about investorshareholder perspectives and make educated and deliberate decisions that are balanced and appropriate for Merck’s diverse shareholder base and in the Company’s best interests. Given our large shareholder base, we concentrate our formal outreach efforts on those of our largest 50 shareholders, who have stewardship teams involved in proxy voting. Our largest 50 shareholders represented approximately 49%50% of our ownership as of December 31, 2022,2023, based on filings made by our shareholders with the SEC on or before March 1, 2023.5, 2024.
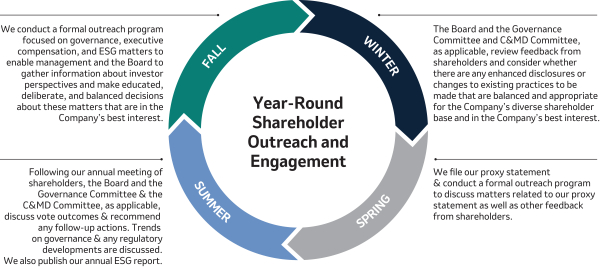
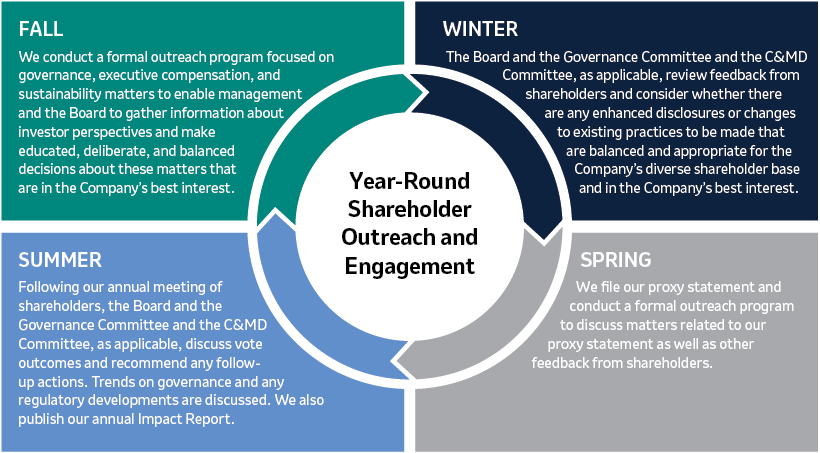
Merck & Co., Inc. 2024 Proxy Statement
Corporate Governance Shareholder Communications with the Board | ÷ ÷ ÷ ÷ | 25 |
In 2022,2023, specifically, we followed this process and held discussions with a number of our shareholders in the spring before the Annual Meeting of Shareholders that year and once again in late fall. Our independent Lead Director, who is also Chair of the Governance Committee, participated in substantive engagements with some of the Company’s shareholders.
Topics Discussed with Shareholders during | ||||
• Company strategy • Board leadership, composition and refreshment • Management succession • Board and management diversity • Human capital management • • Shareholder proposals
| • Global access to Merck products • • Risk oversight • Cybersecurity • Executive compensation programs • Policy and pricing environment • Lobbying expenditures |
• Climate initiatives • Merck Animal Health • Director tenure • Merck culture • Reputation • Board evaluation process | ||
We heard from a number of shareholders that they appreciated the Company’s disclosures of certain demographic information about Directors but also expressed interest in understanding this information on an individualized basis. Following discussion with the Governance Committee and the Board, we added a matrix on page 22 providing demographic information regarding each Director nominee on an individualized basis.
Merck & Co., Inc. 2023 Proxy Statement
|
|
Some key themes emerged as part ofIn addition, we heard that shareholders appreciated the Company’s existing disclosures regarding access and pricing transparency for our various engagements as set forth below.medicines and vaccines, but some were interested in additional disclosure focused on the Company’s approach to patents and access. We included such disclosure on pages 26 to 30.
 What We Heard What We Heard |  What We Did What We Did | |
|
| |
|
| |
Proxy Access
After engaging with a number of our largest shareholders, our Board proactively amended our By-Laws in 2015 to give shareholders a right to proxy access for Director nominations. Our By-Laws allow a shareholder (or a group of no more than twenty shareholders) who has maintained continuous qualifying ownership of at least 3% of the Company’s outstanding common stock for at least three years to include Director nominees constituting up to 20% of the Board in the Company’s proxy materials for an annual meeting of shareholders. Our By-Laws, which prescribe additional requirements for proxy access, are available on our website at www.merck.com/company-overview/leadership/board-of-directors/.
Shareholder Communications with the Board
The Board of Directors welcomes input from shareholders and other interested parties and has established a process to receive these communications. Shareholders and interested parties may communicate directly with the Board, the independent Lead Director, the non-management or independent Directors as a group or other members of the Board by emailing office.secretary@merck.com, or by writing to the following address: Board of Directors, 126 East Lincoln Avenue, P.O. Box 2000, Rahway, N.J. 07065 U.S.A.
In order to manage efficiently the volume of correspondence received, communications will be reviewed by the Office of the Secretary for the purpose of determining whether the contents are appropriate for submission to the entire Board, the Chairman, the independent Lead Director or the Chair of a particular committee. The Office of the Secretary will not transmit:
communications that advocate that the Company engage in illegal activity;
communications that, under community standards, contain offensive or abusive content;
communications that have no relevance to the role of the Board or to the business of the Company;
| • | resumes or other job-related inquiries; and |
mass mailings, solicitations and advertisements.
Merck & Co., Inc. 2024 Proxy Statement
| 26 | ç ç ç ç | Corporate Governance Access and Pricing Transparency for Our Medicines and Vaccines |
Comments or questions regarding the nomination of Directors and other corporate governance matters will be referred to the Chair of the Governance Committee. Comments or questions regarding executive compensation will be referred to the Chair of the C&MD Committee.
In addition, the Audit Committee has established procedures for the receipt, retention and treatment, on a confidential basis, of complaints regarding accounting, internal accounting controls or auditing matters, and the confidential, anonymous submissions by employees of concerns regarding questionable accounting or auditing matters. These procedures are described in the Merck Code of Conduct — Our Values and Standards.
The Merck Code of Conduct is available on our website at www.merck.com/company-overview/culture-and-values/code-of-conduct/values-and-values-and-standards/
standards/
Access and Pricing Transparency for Our Medicines and Vaccines
Our Company
At Merck, our mission is to use the power of leading-edge science to save and improve lives around the world. For more than 130 years, we have worked to help solve the world’s toughest medical challenges. We have developed essential childhood vaccines, introduced the first protease inhibitor, which helped transform AIDS from a death sentence to a chronic disease, and developed the first statin to demonstrate a significant benefit on health outcomes from reducing high cholesterol. More recently, we have been working on the treatment and prevention of cancer. For example, we have been researching and developing KEYTRUDA (pembrolizumab), the Company’s anti-PD-1 (programmed death receptor-1) therapy. Since its first approval in 2014, our Company has demonstrated the efficacy of KEYTRUDA in 39 indications across 17 tumor types and 2 tumor agnostic indications and reached nearly 2 million patients. The impact of KEYTRUDA and other recent treatment advances is difficult to overstate, with a recent American Cancer Society report finding that cancer mortality in the United States has fallen 33% from 1991 to 2021, representing an estimated 4 million Americans whose deaths have been averted.1
Our product GARDASIL is the first FDA-approved vaccine to guard against the human papillomavirus (HPV). HPV is the leading cause of the vast majority of cases of cervical cancer, the fourth most common cancer of women globally. A study of real world evidence published in the New England Journal of Medicine evaluating data from Swedish girls and women between 10 and 30 years of age found a substantially reduced risk of invasive cervical cancer among those who had been fully vaccinated with GARDASIL.2 An American Cancer Society report found similar transformative public health outcomes in the United States, with a 65% decrease in cervical cancer rates in women in their early 20s, following the widespread adoption of HPV vaccines in the United States.3
Our Commitment
We continue to focus on innovative health solutions that advance disease prevention and treatment for both humans and animals and are committed to operating responsibly. This commitment has remained a pillar of strength for us, forming the foundation of our strategic framework and business approach. We are dedicated to putting patients first, upholding ethical standards, demonstrating integrity, showing respect for people, and driving innovation and scientific excellence across all aspects of our operations.
In 2023, we reached more than 500 million people with our innovations across commercial channels, clinical trials, access strategies like our partnership with Gavi and UNICEF, voluntary licensing of antiviral treatments including our investigational antiviral COVID-19 medicine, as well as medicine and vaccine donations.
| 1 | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA: A Cancer J Clin. https://doi.org/10.3322/caac.21820. Published Jan. 17, 2024. Accessed Feb. 3, 2024. |
| 2 | Lei J, Ploner A, Elfstrom E, et al. HPV Vaccination and the Risk of Invasive Cervical Cancer. N Engl J Med 2020; 383:1340-1348. DOI: 10.1056/NENMoa1917338. |
| 3 | Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: A Cancer J Clin. https://doi.org/10.3322/caac.21763. Published Jan. 12, 2023. Accessed Feb. 3, 2024. |
Merck & Co., Inc. 2024 Proxy Statement
Corporate Governance Access and Pricing Transparency for Our Medicines and Vaccines | ÷ ÷ ÷ ÷ | 27 |
We generally report progress on our company-wide Access to Health goals on an annual basis. Our current goals are to:
Reach at least 75 percent of countries in the world annually with our products
Enable access for 350 million more people to our innovative medicines and vaccines through access strategies, solutions, and partnerships by 2025
| • | Advance health equity by reaching 50 million people in low- and middle-income countries (LMICs) and populations underserved by healthcare in high-income countries, by 2025 |
Information regarding our Company’s progress against these goals is available in our most recent annual Impact Report: https://www.merck.com/wp-content/uploads/sites/124/2023/08/Merck-Impact-Report-22-23.pdf.
Pricing Transparency
We have a long history of making our medicines and vaccines accessible and affordable through responsible pricing practices and industry-leading patient-access programs. We are continuing to work to help patients overcome access and affordability challenges.
To provide information about our pricing practices, we have posted on our website an annual Pricing Transparency Report for the United States. The report provides the average annual list price, net price increases and average discounts across the Company’s U.S. portfolio dating back to 2010.
Access & Regulatory
To advance our goal of enabling more people to access our innovative portfolio, we are focusing on building healthcare capacity, strengthening channels for care delivery and fostering sustainable financing. In building healthcare capacity, we help to create greater efficiency for patients in the healthcare system through collaborations with hospitals and healthcare networks. For example, we work with partners using a data-based approach to understand how a patient’s cancer journey can be optimized from earlier diagnosis through to treatment. We also collaborate with different financial institutions and payers, supporting them to expand funding options that help patients and their families cope with potentially high out-of-pocket costs related to critical illness. Through collaborations that reach underserved populations, we are working with healthcare providers and others in the digital health and financial sectors to develop solutions that enhance disease awareness and healthcare access.
We seek to ensure global access to our medicines and vaccines by obtaining and maintaining up-to-date product registrations around the globe. In order to make our products available to the people who need them throughout the world, we registered 156 products and devices in 2022, the majority of which were registered in LMICs in the Asia-Pacific, Central and Eastern Europe, Middle East and Africa, and Americas regions.
We have also taken steps to address the unique needs of LMICs where the infrastructure and personnel necessary to deliver immunization services can be severely limited. Specifically, we have focused on product improvements such as the introduction of vaccine vial monitors for use to assess controlled temperature-chain conditions. In May 2022, we obtained World Health Organization approval for our HPV vaccines’ compatibility for use outside the cold chain for up to four days. This helps enhance accessibility for hard-to-reach populations.
Patient Assistance Program
When market-based solutions are inadequate or unavailable, we pursue programs to provide direct access to our medicines and vaccines, including product donations and patient assistance programs. Individuals who cannot afford our medicines and have no other means of coverage, public or private, may be eligible to be provided with our medicines, at no charge. In the past five years, our patient assistance program in the United States has helped nearly 800,000 patients to obtain our medicines or vaccines free of charge, with an estimated value of $7.8 billion.4
| 4 | Periods prior to June 2021 include products included in spin-off to Organon & Co. |
Merck & Co., Inc. 2024 Proxy Statement
| 28 | ç ç ç ç | Corporate Governance Access and Pricing Transparency for Our Medicines and Vaccines |
Patent Exclusivity and Access
Innovation and access to health are central to our Company’s purpose as a research-intensive biopharmaceutical company. Our Access to Health Guiding Principles, available at https://www.merck.com/wp-content/uploads/sites/124/2021/08/Merck-Access-to-Health-Principles_Update-2021.pdf, guide our global approach to access to health.
We believe intellectual property is an enabler and not a barrier to access to innovative, quality-assured medicines and vaccines. Strong intellectual property systems are the cornerstone of innovation, catalyzing the investment needed to embark on the long, costly, and high-risk process of developing new and improved medicines and vaccines. Intellectual property systems not only reward innovation, but also allow scientists to build on each other’s discoveries and provide a framework for collaboration.
In the pharmaceutical industry, a product’s market exclusivity is generally determined by two forms of intellectual property rights: patents owned or controlled by the innovator company and any forms of regulatory exclusivity to which the innovative medicine is entitled. Patents provide our Company with the right to exclude others from practicing an invention for a limited time. Without a meaningful period of market exclusivity, our Company’s ability to invest in research and development (R&D) to discover and develop promising new medicines may be limited.
The extensive R&D process typically leads to numerous types of innovations associated with a medicine. Patents related to a commercial product may cover, among other things, the product’s active ingredient(s), different uses of the product (such as methods of treating a disease), pharmaceutical formulations directed to specific compositions of active and inactive ingredients within the product, delivery mechanisms, and processes for (or intermediates useful in) the manufacture of the product.
We believe it is important to protect our Company’s ability to innovate by continuing to file for core intellectual property rights that protect important innovations. Because of how the patent system is structured, a patent application typically needs to be filed before the Company will know whether the disclosed invention will be relevant to a product candidate in development, or even to a marketed product, or can effectively evaluate the patient-access situation for the disclosed invention. Often, after a related patent application is filed, years of clinical development are still needed to determine a product candidate’s safety and efficacy and whether or not to pursue regulatory approval and what the access situation may be if regulatory approval were to be pursued and received. As such, our Company evaluates all patent applications and resulting patents annually to assess whether they should be maintained.
Our Company conducts a fact-specific and complicated scientific and legal analysis of new innovations discovered during the R&D process to determine whether or not to pursue patent protection. We evaluate our innovations for potential patenting using similar processes deployed by patent offices when they examine the merits of applications for patentability. Each potential patent application is evaluated separately, including the timing of when and where to file on a particular innovation.
Each patent application is subject to rigorous review by individual patent offices in the countries and/or regions in which it is filed. A patent is granted only when determined to meet the standards of patentability in that country or region. Protection afforded by patents may vary from country to country and depends upon the type of patent and its scope of coverage. In the 2023 10-K, we disclosed the various estimated minimum patent exclusivity dates for each of our key products in the U.S., EU (as specifically represented by France, Germany, Italy and Spain), Japan and China, which is typically the expiration of the patent that claims the active ingredient of the product.
A patent directed to the active ingredient(s) of a potential drug product is typically the first application associated with the potential product to be filed, before any clinical testing occurs. Continued investment in R&D occurs through the drug development process, and often also occurs after the drug product is first commercialized to explore additional potential benefits for patients. For example, additional research may demonstrate that a medicine can also be used to treat different states of the same disease, such as earlier stages of cancer, or to treat completely different conditions, including different forms of cancer or different diseases altogether. In other cases, additional research may lead to a demonstration of increased safety or effectiveness of the medicine, new dosage forms, or new forms of administration of a medicine that can improve patient adherence or convenience, leading to better patient outcomes.
By way of example, since 2014, when KEYTRUDA was first approved to treat advanced or unresectable melanoma, our Company has worked tirelessly to investigate its use in treating additional types of cancers. The FDA requires innovators to
Merck & Co., Inc. 20232024 Proxy Statement
Corporate Governance Access and Pricing Transparency for Our Medicines and Vaccines | ÷ ÷ ÷ ÷ | 29 |
prove their products work by conducting clinical trials for patients having each distinct type of cancer. Each of these clinical trials can take several years to conduct, and there is no guarantee of success. While more than 45 pivotal trials studying KEYTRUDA produced results that enabled the approvals of KEYTRUDA described above, there are more than 25 pivotal trials studying KEYTRUDA that did not produce results supportive of approval.
Between 2011, when our focused KEYTRUDA research program began, and 2023, Merck has invested approximately $30 billion in our own internal clinical development efforts, $14 billion in research collaborations and acquisitions to further the study of KEYTRUDA with other compounds, and $2 billion in capital expenditures to scale up our processes and facilities to manufacture the medicine in large quantities. We expect to invest another $18 billion in KEYTRUDA clinical studies into the 2030s.5
Patents play a key role in safeguarding the investments made in researching and developing additional uses, and our Company prioritizes obtaining intellectual property for those innovations that provide the greatest medical benefit to patients. Importantly, however, patents directed to, for example, additional indications or combination treatments (so-called “secondary or tertiary” patents), which might be added to a product’s label in the future, do not extend the patent life of earlier patents covering the initial innovation associated with a product. This is because each patent has its own respective exclusivity period, which is limited to the specific scope of the patent’s claims. To bring its version of KEYTRUDA to the market, a biosimilar manufacturer does not need to “copy” the subsequent innovation that our Company has made around KEYTRUDA and protected with patents. The FDA enables biosimilar manufacturers to seek approval for fewer than all conditions of use for which the innovator product has been approved. To this end, we continue to expect manufacturers will offer biosimilar options for that initial discovery of pembrolizumab, the active ingredient in KEYTRUDA, by late 2028 when the patent covering the pembrolizumab antibody structure expires.
Access Planning
We build on our legacy of putting patients first by inventing medicines and vaccines to address areas of unmet medical need. This is evident in our strategic priorities that include investing in, augmenting and accelerating our pipeline to deliver life changing products and demonstrating value to our stakeholders and extending access to solutions that address unmet medical needs.
Embedded within our R&D process, we systematically evaluate our candidates to identify the potential to address significant public health burden and unmet medical needs in underserved healthcare settings. This evaluation process informs our product access strategies, with the goal of making our medicines and vaccines available to as many people as possible through sustainable solutions.
To facilitate access, we undertake a systematic evaluation at the onset of Phase 2 clinical studies to determine a candidate’s potential to address unmet medical needs in LMICs. For candidates with significant potential in LMICs as well as settings with underserved populations,6 access planning may start in the pre-clinical phase. Additionally, understanding where health system infrastructure and funding mechanisms are in place is an important component of enabling safe and effective usage, which ultimately facilitates meaningful patient access.
Our R&D Governance Committee is accountable for the evaluation process, and all recommendations are reviewed by our Strategic Policy and Sustainability Council, an internal cross-divisional forum of senior leaders. When a medicine or vaccine candidate with the potential to address significant public health burden in underserved healthcare settings is identified, the access planning process includes engaging all parts of our enterprise, as well as external stakeholders, to identify the optimal solution.
Once approved, we commit to making the product available in all countries where clinical trials have been conducted. Products continue to be evaluated for their potential to address disease burden throughout their life cycle to account for changes in the external environment.
Sometimes the evaluation of a candidate during the R&D process reveals barriers to access in LMICs or underserved settings. In these situations, the evaluation process can inform our approach to strengthening health systems and improving health equity. We recognize that addressing the complex and multi-faceted challenges to accessing healthcare in LMICs requires the collaboration of multiple stakeholders. We actively seek partnerships to achieve solutions that enable access.
| 5 | Figures in this paragraph are rounded. |
| 6 | Underserved populations are defined as those that face health disparities due to disadvantages related to insurance status, social determinants of health, race, ethnicity, gender identity/sexual orientation, age and/ or language preference. |
Merck & Co., Inc. 2024 Proxy Statement
| 30 | ç ç ç ç |
Corporate Governance Political Contributions and Lobbying Expenditure Oversight and Disclosure |
For our manufacturing efforts, this means we are balancing internal and external licensing opportunities, including assessing new voluntary licensing and technology transfer strategies and expanding our network for supply chain design. This business strategy enables enterprise-level strategic segmentation, highlighting the specific access gaps in markets and guiding us to commercially sustainable solutions, including new archetypes for manufacturing and supply execution. Our manufacturing division engages early in the lifecycle of a program through a new product development and commercialization process to understand and facilitate future manufacturing, supply chain and network needs. While not all elements will be known, an early concept of the supply and manufacturing strategy will be critical to preserve future options. This means we start supply chain design early in Phase 1 of clinical development.
Extending access to innovations that address unmet medical need is considered when deciding how to exercise our legitimate intellectual property rights. Future access to our Company’s new medicines and/or new images or uses is considered at multiple stages in a product’s life cycle, including as it approaches the regulatory approval process, after approval of the medicine, and during litigation or any licensing around the product. We only obtain intellectual property by lawful and ethical means, and only enforce those intellectual property rights we believe to be valid. While we continue to believe it is important to protect innovation with intellectual property rights, as a product life-cycle develops, we are deeply committed to making decisions about enforcement or licensing of those rights in a manner consistent with our mission and commitment, including impact on patient access. This may include choosing not to file or enforce our patents in certain parts of the world, partnering with multilateral organizations, non-governmental organizations, and governments to enable greater access in certain markets, particularly in LMICs, and providing financial or free product support through a comprehensive global network of patient assistance programs to qualified patients where allowed by local law. We also support and participate in fair dealing with respect to arms-length, commercial patent settlements entered into with generic manufacturers.
Political Contributions and Lobbying Expenditure Oversight and Disclosure
Merck is committed to participating constructively and responsibly in the political process and to providing clarifying analysis and information regarding the issues that affect our business and patient care. The Company advocates for public policies that foster research into innovative medicines and improve access to medicines, vaccines and health care.healthcare. Our participation in the political process is guided by the following principles: improving patient access to healthcare, including access to medicines and vaccines, improving access to animal health products, and encouraging innovation. The Company’s public policy positions are determined by senior management with oversight by the Governance Committee. Our political contributions are made in accordance with all applicable laws and Company policies and procedures and are overseen by senior management. The Governance Committee monitors all such contributions, and the full Board receives a bi-annual report.
In addition, the Company publicly discloses and regularly updates information regarding its public policy positions and advocacy expenditures on our website at www.merck.com/company-overview/responsibility/transparency-disclosures/. This information includes the Company’s contributions, categorized by state, candidate and amount, for our corporate political and political action committee contributions in the U.S., Canada and Australia. These disclosures include information for the past 5 years. In addition, this information includes a list of U.S. industry and trade groups in which we are members where our dues are greater than $25,000 and the portion of our dues that these groups use for advocacy and/or political activities.
Governance and Transparency around Drug Pricing
In order to provide information about the Company’s pricing practices, the Company annually posts on its website its Pricing Transparency Report for the United States. The report provides the Company’s average annual list price, net price increases and average discounts across the Company’s U.S. portfolio dating back to 2010. In 2022, the Company’s gross U.S. sales were reduced by 39.7% as a result of rebates, discounts and returns. Our process around pricing our products includes regular presentations to the Board on drug pricing strategies. In addition, on balance, over the last few years, our revenue growth has been primarily attributable to increased volume arising from increased demand for our products rather than price increases.
Independence of Directors
The Policies of the Board require that a substantial majority of our Directors be independent. In making independence determinations, the Board observes all relevant criteria established by the U.S. Securities and Exchange Commission (the “SEC”) and the New York Stock Exchange (the “NYSE”), as well as categorical independence standards set forth in the Policies of the Board. The Board considers all relevant facts and circumstances in making an independence determination.
To be considered independent, an outside director must meet the bright line independence tests established by the NYSE, and the Board must affirmatively determine that the director has no direct or indirect material relationship with the Company.
Merck & Co., Inc. 2024 Proxy Statement
Corporate Governance Related Person Transactions | ÷ ÷ ÷ ÷ | 31 |
The Board also rigorously considers all relevant heightened independence requirements for members of the Audit Committee and the C&MD Committee. The Governance Committee reviews the Board’s approach to determining director independence periodically and recommends changes, as appropriate, for consideration and approval by the full Board.
Independence Determinations
In accordance with the NYSE Corporate Governance Listing Standards and the categorical standards reflected in the Policies of the Board, the Board reviewed relationships between the Company and each Director. As a result of that review, the Board has determined that, with the exception of Robert M. Davis, our Chairman, CEO and President, each Director has only immaterial relationships with the Company, and accordingly, each is independent under these standards. The Board also has determined that each member of the Audit Committee, the C&MD Committee and the Governance Committee is independent within the meaning of the NYSE Corporate Governance Listing Standards and the rules of the SEC.
Merck & Co., Inc. 2023 Proxy Statement
|
|
In making these determinations, the Board considered relationships that exist between the Company and other organizations where Directors serve, as well as the fact that in the ordinary course of business, transactions may occur between such organizations and the Company or one of our subsidiaries. The Board also evaluated whether there were any other facts or circumstances that might impair a Director’s independence.
Drs. Lavizzo-Mourey and Seidman are employed at medical or academic institutions with which the Company engages in purchase and/or sale transactions in the ordinary course of business. Dr. Rothman was employed by Johns Hopkins Medicine until July 1, 2022 with which the Company engages in purchase and/or sale transactions in the ordinary course of business. Ms. Coe was employed by Google Inc. until October 3, 2022, and, in 2020, the Company engaged in a purchase transaction with Google Inc. in the ordinary course of business. The Board reviewed transactions with each of these entities and determined that the applicable individual Director had no role with respect to the Company’s decision to make any of the purchases or sales, and the aggregate amounts in each case were less than 2% of the consolidated gross revenues of the other organization and the Company.
Related Person Transactions
Related Person Transaction Policy
The Board has adopted a written Related Person Transaction Policy (the “Policy”) that is incorporated into the Policies of the Board and administered by the Governance Committee. The Policy governs the review and approval of any transactions involving amounts exceeding $120,000 to which the Company or a subsidiary is a party and in which a “related person” has a direct or indirect material interest. A “related person” is any Director, Director nominee, executive officer or holder of more than 5% of any outstanding class of the Company’s voting securities, as well as immediate family members or certain affiliated entities of any of the foregoing persons.
Pursuant to the Policy, management determines whether a transaction requires review by the Governance Committee, in which case the transaction, along with all material information, will be disclosed to the Governance Committee for review, approval, ratification or termination. In the event a related person transaction is approved by the Governance Committee, such transaction will be subject to ongoing monitoring to ensure that the transaction remains fair and reasonable to the Company. For additional information, the full Policy is available on the Company’s website at www.merck.com/company-overview/leadership/board-of-directors/ in the Policies of the Board.
Certain Related Person Transactions
Each Director and executive officer of Merck annually, and each Director nominee before such nominee’s nomination, completes and submits to the Company a Director & Officer (“D&O”) Questionnaire. The D&O Questionnaire requests, among other things, information regarding whether any Director, Director nominee, executive officer or their immediate family members had an interest in any transaction or proposed transaction with Merck or its subsidiaries or has a relationship with a company that has entered or proposes to enter into such a transaction.
After review of the D&O Questionnaires by the Office of the Secretary, the responses are collected, summarized and distributed to responsible areas within the Company to identify any potential transactions. All relevant relationships and any transactions, along with payables and receivables, are compiled for each person and affiliation. Management submits a report of the affiliations, relationships, transactions and appropriate supplemental information to the Governance Committee for its review. Based on this information for 2022,2023, the Governance Committee has determined that no transactions require disclosure under Item 404(a) of SEC Regulation S-K.
Merck & Co., Inc. 20232024 Proxy Statement
| 32 | ç ç ç ç |
Corporate Governance Compensation Consultants |
Compensation Consultants
Role of Compensation Consultants
The C&MD Committee retains the services of a compensation consultant to serve as an objective third-party advisor on the reasonableness of compensation levels and on the appropriateness of the compensation program structure in supporting our business strategy and human resource objectives. Since 2008, the C&MD Committee has retained FW Cook as its compensation consultant. In addition, the Governance Committee has retained, and may in the future retain FW Cook to assist with a review of the Directors’ compensation program.
Independence of the Compensation Consultant
The C&MD Committee annually reviews the services provided by FW Cook and has concluded that FW Cook is independent in providing executive compensation consulting services. The C&MD Committee evaluated its relationship with FW Cook in 20222023 and determined that FW Cook’s work for the C&MD Committee did not raise any conflicts of interest, consistent with the guidance provided under the Dodd-Frank Act and by the SEC and the NYSE. In making this determination, the C&MD Committee reviewed information provided by FW Cook on the following factors:
In particular, the C&MD Committee noted that (i) FW Cook provided no other services to Merck; and (ii) FW Cook’s work is performed directly on behalf of the Board working in cooperation with management, to assist both the C&MD Committee and the Governance Committee, as applicable, with executing their respective responsibilities.
Services Performed During 20222023
During 2022,2023, FW Cook supported the C&MD Committee in the following areas:
Since 2010, management has retained Pay Governance LLC to provide consulting services on an as-needed basis. In November 2022,Although Pay Governance performed adid not provide any services during 2023, they will perform their biennial risk assessment of our compensation programs. This report was reviewed by FW Cook and presented to the C&MD Committee. The assessment indicated that our compensation programs do not create incentives for excessive risk-taking and include meaningful safeguards to mitigate compensation program risk.in November 2024.
Merck & Co., Inc. 20232024 Proxy Statement
| ÷ ÷ ÷ ÷ | 33 |
Stock Ownership Information
Stock Ownership of Directors and Officers
The table below reflects the number of shares of Merck common stock beneficially owned by (a) each of our Directors; (b) each of our executive officers named in the Summary Compensation table; and (c) all Directors and executive officers as a group. These numbers are rounded to the nearest whole share. As of February 28, 2023, 2,538,433,11129, 2024, 2,533,298,070 shares of Merck common stock were issued and outstanding. Unless otherwise noted, the information is stated as of February 28, 2023,29, 2024, and the beneficial owners exercise sole voting and/or investment power over their shares. In addition, unless otherwise indicated, the address for each person named below is c/o Merck & Co., Inc., 126 East Lincoln Avenue, Rahway, New Jersey 07065.
| Company Common Stock | Company Common Stock | |||||||||||||||||||||||||||
Name of Beneficial Owner | Shares Beneficially Owned(1) | Right to Acquire Beneficial Ownership Under Options/Stock Units Exercisable/Distributable Within 60 Days(2) | Percent of Class | Phantom Stock Units(3) | Shares Beneficially Owned(1) | Right to Acquire Beneficial Ownership Under Options/Stock Units Exercisable/Distributable Within 60 Days(2) | Percent of Class | Phantom Stock Units(3) | ||||||||||||||||||||
Robert M. Davis | 271,817 | 439,473 | * | — | ||||||||||||||||||||||||
Robert M. Davis | ||||||||||||||||||||||||||||
Robert M. Davis | ||||||||||||||||||||||||||||
Robert M. Davis | 348,327 | 275,441 | * | — | ||||||||||||||||||||||||
Douglas M. Baker, Jr. | ||||||||||||||||||||||||||||
Douglas M. Baker, Jr. | ||||||||||||||||||||||||||||
Douglas M. Baker, Jr. | ||||||||||||||||||||||||||||
Douglas M. Baker, Jr. | 1,000 | — | * | 2,416 | 1,000 | — | * | 4,502 | ||||||||||||||||||||
Mary Ellen Coe | 10 | — | * | 17,195 | ||||||||||||||||||||||||
Mary Ellen Coe | ||||||||||||||||||||||||||||
Mary Ellen Coe | ||||||||||||||||||||||||||||
Mary Ellen Coe | 10 | — | * | 20,822 | ||||||||||||||||||||||||
Pamela J. Craig | ||||||||||||||||||||||||||||
Pamela J. Craig | ||||||||||||||||||||||||||||
Pamela J. Craig | ||||||||||||||||||||||||||||
Pamela J. Craig | 1,715 | — | * | 23,663 | 1,715 | — | * | 26,323 | ||||||||||||||||||||
Thomas H. Glocer | 5,100 | — | * | 86,986 | ||||||||||||||||||||||||
Thomas H. Glocer | ||||||||||||||||||||||||||||
Thomas H. Glocer | ||||||||||||||||||||||||||||
Thomas H. Glocer | 5,100 | — | * | 93,087 | ||||||||||||||||||||||||
Risa J. Lavizzo-Mourey | ||||||||||||||||||||||||||||
Risa J. Lavizzo-Mourey | ||||||||||||||||||||||||||||
Risa J. Lavizzo-Mourey | ||||||||||||||||||||||||||||
Risa J. Lavizzo-Mourey | 1,000 | — | * | 8,067 | 1,000 | — | * | 10,306 | ||||||||||||||||||||
Stephen L. Mayo | 100 | — | * | 5,415 | ||||||||||||||||||||||||
Stephen L. Mayo | ||||||||||||||||||||||||||||
Stephen L. Mayo | ||||||||||||||||||||||||||||
Stephen L. Mayo | 100 | — | * | 7,582 | ||||||||||||||||||||||||
Paul B. Rothman | ||||||||||||||||||||||||||||
Paul B. Rothman | ||||||||||||||||||||||||||||
Paul B. Rothman | ||||||||||||||||||||||||||||
Paul B. Rothman | 100 | — | * | 23,672 | 100 | — | * | 26,323 | ||||||||||||||||||||
Patricia F. Russo | 13,148 | — | * | 48,556 | ||||||||||||||||||||||||
Patricia F. Russo | ||||||||||||||||||||||||||||
Patricia F. Russo | ||||||||||||||||||||||||||||
Patricia F. Russo | 13,148 | — | * | 51,887 | ||||||||||||||||||||||||
Christine E. Seidman | ||||||||||||||||||||||||||||
Christine E. Seidman | ||||||||||||||||||||||||||||
Christine E. Seidman | ||||||||||||||||||||||||||||
Christine E. Seidman | 100 | — | * | 10,263 | 100 | — | * | 12,859 | ||||||||||||||||||||
Inge G. Thulin | 100 | — | * | 16,883 | ||||||||||||||||||||||||
Inge G. Thulin | ||||||||||||||||||||||||||||
Inge G. Thulin | ||||||||||||||||||||||||||||
Inge G. Thulin | 100 | — | * | 19,360 | ||||||||||||||||||||||||
Kathy Warden | 500 | — | * | 8,203 | ||||||||||||||||||||||||
Kathy Warden | ||||||||||||||||||||||||||||
Kathy Warden | ||||||||||||||||||||||||||||
Kathy Warden | 500 | — | * | 10,437 | ||||||||||||||||||||||||
Peter C. Wendell | 1,000 | — | * | 118,196 | ||||||||||||||||||||||||
Peter C. Wendell(4) | ||||||||||||||||||||||||||||
Peter C. Wendell(4) | ||||||||||||||||||||||||||||
Peter C. Wendell(4) | ||||||||||||||||||||||||||||
Peter C. Wendell(4) | 1,000 | — | * | 124,525 | ||||||||||||||||||||||||
Kenneth C. Frazier(4) | 705,220 | 1,918,034 | * | — | ||||||||||||||||||||||||
Sanat Chattopadhyay | ||||||||||||||||||||||||||||
Sanat Chattopadhyay | ||||||||||||||||||||||||||||
Sanat Chattopadhyay | ||||||||||||||||||||||||||||
Sanat Chattopadhyay | 157,192 | 232,394 | * | 14,803 | ||||||||||||||||||||||||
Chirfi Guindo | 55 | — | * | — | ||||||||||||||||||||||||
Richard DeLuca | ||||||||||||||||||||||||||||
Richard DeLuca | ||||||||||||||||||||||||||||
Richard DeLuca | ||||||||||||||||||||||||||||
Richard DeLuca | 137,013 | 212,224 | * | — | ||||||||||||||||||||||||
Dean Li | ||||||||||||||||||||||||||||
Dean Li | ||||||||||||||||||||||||||||
Dean Li | ||||||||||||||||||||||||||||
Dean Li | 19,933 | 95,394 | * | — | 46,242 | 145,789 | * | — | ||||||||||||||||||||
Caroline Litchfield | 31,252 | 183,515 | * | — | ||||||||||||||||||||||||
Caroline Litchfield | ||||||||||||||||||||||||||||
Caroline Litchfield | ||||||||||||||||||||||||||||
Caroline Litchfield | 50,908 | 192,933 | * | — | ||||||||||||||||||||||||
Jennifer Zachary | 53,920 | — | * | — | ||||||||||||||||||||||||
All Directors and Executive Officers as a Group (28 individuals) | 1,542,711 | 3,513,212 | * | 384,319 | ||||||||||||||||||||||||
All Directors and Executive Officers as a | ||||||||||||||||||||||||||||
All Directors and Executive Officers as a | ||||||||||||||||||||||||||||
All Directors and Executive Officers as a | ||||||||||||||||||||||||||||
All Directors and Executive Officers as a | 926,956 | 1,411,677 | * | 422,816 | ||||||||||||||||||||||||
| * | Less than 1% of the Company’s outstanding shares of common stock. |
| (1) | Includes equivalent shares of common stock held by the Trustee of the Merck U.S. Savings Plan, for the accounts of individuals as follows: Mr. |
| (2) | This column reflects the number of shares that could be acquired within 60 days of February |
| (3) | Represents phantom shares denominated in Merck common stock under the Plan for Deferred Payment of Directors’ Compensation or the Merck Deferral Program. |
| (4) | Mr. |
Merck & Co., Inc. 20232024 Proxy Statement
| 34 | ç ç ç ç |
Stock Ownership Information Delinquent Section 16(a) Reports |
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act, as amended, requires our executive officers, directors, and beneficial owners of more than 10% of our common stock to file stock ownership reports and reports of changes in ownership with the SEC. Based on a review of those reports and written representations from the reporting persons, to our knowledge, all such reports for 20222023 were filed on a timely basis, except one Form 3/A by Chirfi Guindo filed on April 28, 2023 solely to restate the number of shares beneficially and directly owned by Mr. Guindo, and one Form 4 by Kenneth C. Frazier reporting a special one-time Restricted Stock Unit grant that was due on August 5, 2022, butJoseph Romanelli filed on August 19, 2022.June 30, 2023 solely to report purchases and sales that were not directed by Mr. Romanelli, but were rather executed by a brokerage firm for the benefit of Mr. Romanelli’s managed account without his knowledge.
Stock Ownership of Certain Beneficial Owners
The table below reflects the number of shares beneficially owned by persons or entities known to us to own more than 5% of the outstanding shares of Merck common stock as of December 31, 2022.2023. As of December 31, 2022, 2,537,840,1082023, 2,531,633,273 shares of Merck common stock were issued and outstanding.
Name and Address of Beneficial Owner | Amount and Nature of Beneficial Ownership | Percent of Class | |||||||||||
The Vanguard Group 100 Vanguard Blvd., Malvern, PA 19355 | |||||||||||||
BlackRock, Inc. 55 East 52nd Street, New York, NY 10055 | |||||||||||||
| (1) | As reported on Amendment No. |
| (2) | As reported on Amendment No. |
Merck & Co., Inc. 20232024 Proxy Statement
| ||||||||
Proposal 1 Election of Directors | | | | | | 35 |
The Board has recommended 1312 nominees for election as Directors at the 20232024 Annual Meeting of Shareholders:Meeting: Mr. Douglas M. Baker, Jr., Ms. Mary Ellen Coe, Ms. Pamela J. Craig, Mr. Robert M. Davis, Mr. Thomas H. Glocer, Dr. Risa J. Lavizzo-Mourey, Dr. Stephen L. Mayo, Dr. Paul B. Rothman, Ms. Patricia F. Russo, Dr. Christine E. Seidman, Mr. Inge G. Thulin, and Ms. Kathy J. Warden and Mr. Peter C. Wendell.Warden. All nominees, other than Mr. Davis, our Chairman and Chief Executive Officer, satisfy the NYSE independence requirements. Mr. Peter Wendell will be retiring from the Board effective as of the 2024 Annual Meeting. Mr. Wendell has a distinguished history of insightful contributions since joining the Board in 2003, and the Board has benefited from his extensive management, financial and venture capital expertise.
All Director nominees currently serve on the Board and were elected by the shareholders at the 20222023 Annual Meeting.Meeting of Shareholders.
In addition, all Director nominees named in this proxy statement meet the Board’s criteria for membership and were recommended by the Governance Committee, and approved by the Board, for election by shareholders at the 20232024 Annual Meeting. All of them hold, or have held, senior leadership positions in large, complex organizations, including multi-national corporations, medical or academic institutions, or charitable organizations. In these positions, our Director nominees have demonstrated their leadership, intellect and analytical skills and gained deep experience in core disciplines significant to their oversight responsibilities at Merck.the Company. Their varied roles and experiences reflect a diversity of perspectives, skills and expertise to address the Company’s current and anticipated needs as the Company’s opportunities and challenges evolve. If elected, each nominee will serve until the 20242025 Annual Meeting of Shareholders or until a successor has been duly elected and qualified, subject to their earlier resignation, death or removal.
Any Director nominee who does not receive a majority of the votes cast with respect to his or her election will not be re-elected as a Director of the Company. However, under the New Jersey Business Corporation Act, incumbent Directors who are not re-elected in an uncontested election because of a failure to receive a majority of the votes cast in favor of their re-election will be “held over” and continue as Directors of the Company until they resign, or their successors are elected at the next election of directors. Our Incumbent Director Resignation Policy, included in the Policies of the Board, provides that an incumbent Director who is not re-elected must promptly submit a resignation. The Governance Committee will evaluate whether to accept such resignation and make a recommendation to the full Board. The Board must act on the recommendation no later than 90 days following certification of the shareholder vote and publicly disclose its decision and rationale.
If any Director nominee becomes unavailable for election (which we do not expect), votes will be cast for such substitute Director nominee or nominees as may be designated by the Board, unless the Board reduces its size.
There are no family relationships among Merck’sthe Company’s executive officers and Directors.
We provide below biographical information for each Director nominee, including key experience, qualifications and skills such Director nominee contributes to the Board in light of our current needs and business priorities.
FOR
The Board of Directors recommends that the shareholders vote FOR the election of each of the Director Nominees.
|
Merck & Co., Inc. 20232024 Proxy Statement
| 36 | ç ç ç ç |
Proposal 1 Election of Directors |
|
Douglas M. Baker, Jr. Independent
|
| ||||||||||
Age:
| ||||||||||||
Director Since: 2022
| ||||||||||||
Committees:
| ||||||||||||
 |  | |||||||||||
Audit
|
Governance | |||||||||||
Experience
|
Mr. Baker has wide-ranging expertise in corporate governance and general and organizational management, including a deep understanding of global marketing, sales and operations of public companies. Mr. Baker is a Founding Partner of E2SG Partners, a company that invests in environmentally sustainable technologies. Previously, Mr. Baker was Chairman & Chief Executive Officer of Ecolab Inc., a provider of water and hygiene services and technologies for the food, hospitality, industrial and energy markets. Mr. Baker is also a member of the Board of Target Corporation and served as their Lead Independent Director from 2015 to 2021. This directorship as well as his previous directorship at U.S. Bancorp provide him with deep experience on governance issues facing large public companies. |
Career Highlights
|
E2SG Partners • Founding Partner (2022-Present) Ecolab Inc. • Executive Chairman (2021-2022) • Chairman and Chief Executive Officer (2006-2020) • Chief Executive Officer (2004-2006)
|
Other Public Directorships
|
Current • Target Corporation (Since 2013) Former • Ecolab Inc. (2006-2022) • U.S. Bancorp (2008-2018)
|
|
Mary Ellen Coe Independent
|
| ||||||||
Age:
| ||||||||||
Director Since: 2019
| ||||||||||
Committees:
| ||||||||||
 |  | |||||||||
Compensation and Management Development
|
Research
| |||||||||
Experience
|
Ms. Coe has a deep understanding of the digital, media and technology landscape, as well as global strategy and operations, due to her experience as a senior leader at YouTube Inc. and Google Inc. At YouTube, Ms. Coe currently serves as the Chief Business Officer, leading all global business operations, content plus distribution partnerships across multiple areas and YouTube’s monetization ecosystem, including streaming businesses like YouTube TV, YouTube Music and YouTube Premium, in addition to supporting the company’s advertising business. At Google, Ms. Coe previously served as the President of Google Customer Solutions, overseeing the global ads business for mid-market and small businesses, serving millions of customers and thousands of partners worldwide. She also has extensive marketing and sales expertise from her leadership position at McKinsey and other global marketing consulting firms. |
Career Highlights
|
YouTube Inc. • Chief Business Officer (2022-Present) Google Inc. • President, Google Customer Solutions (2017-2022) • Vice President, Go-to-Market Vice President, Go-to-Market Operations and Strategy (2012-2017)
|
Other Public Directorships
|
Current • None Former • Whole Foods Market, Inc. (2016-2017)
|
Merck & Co., Inc. 20232024 Proxy Statement
Proposal 1 Election of Directors | ÷ ÷ ÷ ÷ | 37 |
|
Pamela J. Craig Independent
|
| ||||||||
Age:
| ||||||||||
Director Since: 2015
| ||||||||||
Committees:
| ||||||||||
 |  | |||||||||
Audit (Chair)
|
Governance
| |||||||||
Experience
|
Ms. Craig has extensive finance, management, operational, technology and international business expertise, including her history of accomplishment and executive ability as Chief Financial Officer of Accenture plc. In addition, her directorships at other public companies, including her service as a member of the Audit Committee and Chair of the Compensation & Talent Committee of 3M Company, as a member of the Audit and Corporate Responsibility and Sustainability Committees of Corning Incorporated, and as chair of the Technology Committee and a member of the Compensation Committee of Progressive Insurance, provide her with valuable experience on governance issues facing public companies. |
Career Highlights
|
Accenture plc, global management consulting, technology services and outsourcing company • Chief Financial Officer (2006-2013) • Senior Vice President, Finance • Group Director, Business Operations and Services (2003-2004) • Managing Partner, Global Business Operations (2001-2003)
|
Other Public Directorships
|
Current • Progressive Insurance (since 2018) •
Former • 3M Company (2019-2023) • Akamai Technologies, Inc. (2011-2019) • Wal-Mart Stores, Inc. (2013-2017)
|
|
|
Robert M. Davis Management
|
| ||||||||
Age:
| ||||||||||
Director Since: 2021
| ||||||||||
| ||||||||||
| ||||||||||
Experience
|
Mr. Davis, Merck’s Chairman, Chief Executive Officer and President, has extensive management, financial, and operational expertise. Previously, Mr. Davis served as Merck’s President, with responsibility for Merck’s operating divisions, Human Health, Animal Health, Manufacturing and Merck Research Laboratories. Prior to that, he served as Merck’s Chief Financial Officer and Executive Vice President, Global Services, with broad responsibilities, including with respect to finance, risk management, real estate operations, corporate strategy, business development, information technology and procurement. In addition, Mr. Davis’s service on the board of directors of Duke Energy, including his roles as Chair of the Finance & Risk Management Committee and member of the Corporate Governance Committee, has provided him with valuable experience on governance issues facing public companies. Prior to joining Merck in 2014, Mr. Davis held leadership roles at Baxter International, Inc., including as Corporate Vice President and President of Medical Products and Corporate Vice President and Chief Financial Officer. |
Career Highlights
|
Merck & Co., Inc. • Chairman (2022-present) • Chief Executive Officer and President (2021-present) • Chief Financial Officer and Executive Vice President, Global Services (2016-2021) • Chief Financial Officer and Executive Vice President (2014-2016) Baxter International, Inc. • Corporate Vice President and President, Medical Products (2010-2014) • Corporate Vice President and Chief Financial Officer (2006-2010) • Corporate Vice President and Treasurer (2004-2006)
|
Other Public Directorships
|
Current • Duke Energy (since 2018) Former • C.R. Bard (2015-2017)
|
Merck & Co., Inc. 20232024 Proxy Statement
| 38 | ç ç ç ç |
Proposal 1 Election of Directors |
|
Thomas H. Glocer Independent Lead Director
|
| ||||||||
Age:
| ||||||||||
Director Since: 2007
| ||||||||||
Committees:
| ||||||||||
 |  | |||||||||
Compensation and Management Development
|
Governance (Chair) | |||||||||
| ||||||||||
Experience
|
Mr. Glocer has extensive management, operational, technology and international business expertise, including his history of accomplishment and executive ability as CEO and a Director of Thomson Reuters Corporation. In addition, his directorships at other public companies, including his service as Lead Director and as a member of the |
Career Highlights
|
Angelic Ventures LP, a family office investing in early-stage technology and data companies • Founder and Managing Partner (2012-present) Thomson Reuters Corporation, multi-national media and information firm • Chief Executive Officer (2008-2011) • Chief Executive Officer, Reuters Group PLC (2001-2008)
|
Other Public Directorships
|
Current • Morgan Stanley (since 2013) • Publicis Groupe (since 2016) Former • None
|
|
Risa J. Lavizzo-Mourey, M.D. Independent
|
| ||||||||
Age:
| ||||||||||
Director Since: 2020
| ||||||||||
Committees:
| ||||||||||
|
| |||||||||
Compensation and Management Development
| Research | |||||||||
| ||||||||||
Experience
|
Dr. Lavizzo-Mourey has extensive health policy experience, serving as Robert Wood Johnson Foundation Population Health and Health Equity Professor Emerita and formerly as President and Chief Executive Officer of Robert Wood Johnson Foundation, the nation’s largest healthcare-focused philanthropic organization. Her role at Robert Wood Johnson Foundation provided her with deep management, strategic, human capital and talent development expertise. In addition, her directorships at other public companies, including her service as Lead Director and Chair of the Governance Committee
|
Career Highlights
|
University of Pennsylvania • Robert Wood Johnson Foundation Population Health and Health Equity Professor Emerita (2021-Current) • Penn Integrates Knowledge Professor of Health Equity and Health Policy Robert Wood Johnson Foundation • President Emerita (2017-present) • President and Chief Executive Officer (2003-2017) • Senior Vice President and Director (2001-2002)
|
Other Public Directorships
|
Current • GE HealthCare Technologies, Inc. (since 2023) • Intel Corporation (since 2018) Former • Better Therapeutics
• General Electric Company (2017-2023) • Hess Corporation (2004-2020)
|
Merck & Co., Inc. 20232024 Proxy Statement
Proposal 1 Election of Directors | ÷ ÷ ÷ ÷ | 39 |
|
Stephen L. Mayo, Ph.D. Independent
|
| ||||||||
Age:
| ||||||||||
Director Since: 2021
| ||||||||||
Committees:
| ||||||||||
 |  | |||||||||
Audit |
Research | |||||||||
| ||||||||||
Experience
|
Dr. Mayo has extensive scientific experience relevant to the biopharmaceutical industry, including being the Bren Professor of Biology and Chemistry, Merkin Institute Professor and former Chair of the Division of Biology and Biological Engineering at the California Institute of Technology (“Caltech”) and co-founder of Xencor, a public antibody engineering company. In addition, in his role as the former Vice Provost at Caltech, Dr. Mayo oversaw Caltech’s technology licensing program. Elected to the National Academy of Sciences in 2004 for his pioneering contributions in the field of protein design, Dr. Mayo has also served as a presidential appointee on the National Science Foundation’s National Science Board and as an elected board member for the American Association for the Advancement of Science. Dr. Mayo also serves as a member of the board of directors of Sarepta Therapeutics, Inc and Allogene Therapeutics, Inc. |
Career Highlights
|
California Institute of Technology • Merkin Institute Professor (2021-present) • Bren Professor of Biology and Chemistry (2007–present) • Chair, Division of Biology and Biological Engineering (2010–2020) • Vice Provost for Research (2007–2010) Howard Hughes Medical Institute, non-profit medical research organization • Investigator (1994–2007)
|
Other Public Directorships
|
Current • Allogene Therapeutics (since 2021) • Sarepta Therapeutics (since 2021) Former • None
|
|
Paul B. Rothman, M.D. Independent
|
| ||||||||
Age:
| ||||||||||
Director Since: 2015
| ||||||||||
Committees:
| ||||||||||
 |
| |||||||||
Audit | Research (Chair)
| |||||||||
Experience
|
Dr. Rothman has extensive expertise in patient care, science and medicine relevant to the pharmaceutical industry, including through his past experiences as (a) the CEO of Johns Hopkins Medicine and the Dean of Medical Faculty and Vice President for Medicine, The Johns Hopkins University, and (b) Dean and Head of Internal Medicine at Carver College of Medicine at the University of Iowa. His vast operational and management experience leading a large-scale medical organization provide him with a deep understanding of the complexities of the U.S. healthcare delivery system and policy environment. |
Career Highlights
|
Johns Hopkins University • Dean of the Medical Faculty and Vice President for Medicine (2012-2022) Johns Hopkins Medicine • Chief Executive Officer (2012-2022) Carver College of Medicine at the University of Iowa • Dean (2008-2012) • Head of Internal Medicine (2004-2008)
|
Other Public Directorships
|
Current • Former • None
|
Merck & Co., Inc. 20232024 Proxy Statement
| 40 | ç ç ç ç |
Proposal 1 Election of Directors |
|
Patricia F. Russo Independent
|
| ||||||||
Age:
| ||||||||||
Director Since: 1995(1)
| ||||||||||
Committees:
| ||||||||||
 |  | |||||||||
Compensation and Management Development (Chair)
|
Governance | |||||||||
Experience
|
Ms. Russo has extensive management, operational, international business and financial expertise, as well as a broad understanding of the technology industry, which includes her career achievements during her tenure as CEO and Director of Alcatel-Lucent and Lucent Technologies Inc. In addition, her directorships at other public companies, including her roles as the Non-executive
|
Career Highlights
|
Hewlett Packard Enterprise Company, technology company • Non-executive Chair (2015-present) Alcatel-Lucent, global telecommunications equipment company • Chief Executive Officer and Director (2006-2008) • Chairman, Lucent Technologies Inc. (2003-2006) • President and Chief Executive Officer, Lucent Technologies Inc. (2002-2006)
|
Other Public Directorships
|
Current • General Motors Company (since 2009), Independent Lead Director (2010-2014; 2021-present) • Hewlett Packard Enterprise Company (since 2015), Non-executive • KKR Management Inc. (the managing partner of KKR & Co., L.P.) (since 2011) Former • Arconic, Inc. (2016-2018) formerly Alcoa, Inc. (2008-2016)
|
| (1) | Ms. Russo was on the Board of Directors of Schering-Plough Corporation from 1995 until 2009 when the Company became Merck & Co., Inc. |
|
Christine E. Seidman, M.D. Independent
|
| ||||||||
Age:
| ||||||||||
Director Since: 2020
| ||||||||||
Committees:
| ||||||||||
 |  | |||||||||
Audit |
Research
| |||||||||
| ||||||||||
Experience
|
Dr. Seidman has extensive scientific experience relevant to the biopharmaceutical industry, including being the Thomas W. Smith Professor of Medicine and Genetics at Harvard Medical School and the director of the Cardiovascular Genetics Center. Her role leading the Seidman Laboratory, a research laboratory focusing on integrating clinical medicine and molecular technologies to define disease-causing gene mutations and genetic variations that increase disease risk, provides Dr. Seidman with managerial experience relevant to scientific research. The recipient of many honors, Dr. Seidman was elected to the American Society for Clinical Investigation, the National Academy of Sciences, American Academy of Arts and Sciences and the National Academy of Medicine.
Awards • The Ray C. Fish Award for Scientific Achievement (2020) • American Heart Association Medal for Genomic and Precision Medicine (2019) • Vanderbilt Prize in Biomedical Sciences (2019) |
Career Highlights
|
Harvard Medical School/Brigham and Women’s Hospital (Harvard University) • Thomas W. Smith Professor of Medicine and Genetics (2005-present) • Professor of Genetics and Medicine (1998-2005) • Professor of Medicine (1997-1998) Howard Hughes Medical Institute, non-profit medical research organization • Investigator (1994-present) Brigham and Women’s Hospital • Director, Cardiovascular Genetics Center (1992-present) • Attending Physician, Cardiovascular Division (1987-present)
|
Other Public Directorships
|
Current • None Former • None
|
Merck & Co., Inc. 20232024 Proxy Statement
Proposal 1 Election of Directors | ÷ ÷ ÷ ÷ | 41 |
|
Inge G. Thulin Independent
|
| ||||||||
Age:
| ||||||||||
Director Since: 2018
| ||||||||||
Committees:
| ||||||||||
 |  | |||||||||
Compensation and Management Development
|
Governance | |||||||||
| ||||||||||
Experience
|
Mr. Thulin has extensive management, operational, technology and international business expertise, as demonstrated by a track record of success leading 3M Company. Mr. Thulin possesses broad industry experience drawn from 3M’s diverse businesses, commitment to research and strong life sciences division. He also brings valuable insight into driving innovation, based on his experience with new product development and manufacturing. In addition, his previous directorships at other public companies provide him with deep experience on governance issues facing large public companies. |
Career Highlights
|
3M Company, global technology company • Executive Chairman (2018-2019) • Chairman, President and Chief Executive Officer (2012-2018) • President and Chief Executive Officer (2012) • Executive Vice President and Chief Operating Officer (2011-2012) • Executive Vice President, International Operations (2004-2011)
|
Other Public Directorships
|
Current • None Former • 3M Company (2012-2019) • Chevron Corporation (2015-2019)
|
|
Kathy J. Warden Independent
|
| ||||||||
Age:
| ||||||||||
Director Since: 2020
| ||||||||||
Committees:
| ||||||||||
 |  | |||||||||
Audit |
Governance | |||||||||
Experience
|
Ms. Warden has broad experience in operational leadership as Chair, CEO and President of Northrop Grumman Corporation, an innovative company using science, technology and engineering to create and deliver products and services. Ms. Warden has extensive expertise in strategy, performance and business development in government and commercial markets, as well as cybersecurity expertise. Prior to joining Northrop Grumman, Ms. Warden held leadership roles at General Dynamics and General Electric. In addition, Ms. Warden is a former chair of the board of the Richmond Federal Reserve Bank. |
Career Highlights
|
Northrop Grumman Corporation,global security company • Chair, Chief Executive Officer and President (2019-present) • President and Chief Operating Officer (2018) • Corporate Vice President and President, Mission System Sector (2016-2017) • Corporate Vice President and President, Information Systems Sector (2013-2015) • Vice President, Cyber Intelligence Division (2011-2012)
|
Other Public Directorships
|
Current • Northrop Grumman Corporation Former • None
|
Merck & Co., Inc. 20232024 Proxy Statement
| 42 |
|
|
| ||||||||||
| ||||||||||
| ||||||||||
| ||||||||||
 |  | |||||||||
| ||||||||||
|
|
|
|
|
|
Merck & Co., Inc. 2023 Proxy Statement
|
|
Director Compensation
Our non-employee Directors receive cash compensation, as well as cash-settled equity compensation in the form of deferred stock units, for their Board service. During 2022,2023, non-employee Directors were compensated for their Board service as shown in the chart below.
20222023 Schedule of Director Fees
Compensation Element(1) | Director Compensation Program | |
Annual Retainer | $120,000 | |
Annual Mandatory Deferral | $220,000 credit to Director’s Merck common stock account under the Plan for Deferred Payment of Directors’ Compensation | |
Committee Chair Retainer | $30,000 for the Audit Committee(2) | |
$20,000 for the Governance Committee(3) | ||
$20,000 for the Compensation and Management Development Committee | ||
$20,000 for the Research Committee | ||
Audit Committee Member Retainer | $10,000(2) | |
Lead Director Retainer | $40,000(3) |
| (1) | All compensation is annual. Retainers are paid in quarterly installments and may be voluntarily deferred at the Director’s election. |
| (2) | The Audit Committee Chair retainer includes the Audit Committee Member retainer fee in the amount of $10,000. |
| (3) | The Lead Director is the Chair of the Governance Committee as prescribed by the Governance Committee charter. As a result of the combined responsibility, the Lead Director retainer totals $60,000 in the aggregate. |
Directors’ Deferral Plan
Annual Retainer
Under the Merck & Co., Inc. Plan for Deferred Payment of Directors’ Compensation (“Directors’ Deferral Plan”), each Director may elect to defer all or a portion of cash compensation from retainers. Any amount so deferred is, at the Director’s election, valued as if invested in investment measures offered under the Merck U.S. Savings Plan, including our common stock, and is payable in cash installments or as a lump sum generally no sooner than one year after service as a Director ceases.
Annual Mandatory Deferral
In addition to the annual retainer, upon election (or re-election) at thean Annual Meeting of Shareholders, each Director receives a credit, which for 2022,2023, was valued at $220,000 in the form of phantom shares denominated in Merck common stock to the Director’s account under the Directors’ Deferral Plan. Directors who join the Board after thean Annual Meeting of Shareholders are credited with a pro-rata portion. All distributions from the Directors’ deferred account are payable in cash installments or as a lump sum and are generally made no sooner than one year after service as a Director ceases.
Expenses and Matching Gift Program
We reimburse all Directors for travel and other necessary business expenses incurred in the performance of their
services for us. We also extend coverage to Directors under our travel accident and directors’ and officers’ indemnity insurance policies. Directors are also eligible to participate in the Merck Foundation Matching Gift Program. The maximum gift total for an active Director participating in the matching gift program is $30,000 in any calendar year.
Director Stock Ownership Guidelines
Upon joining the Board, each Director must own at least one share of Merck common stock. Directors must attain a target Merck common stock ownership level having a value equal to five times the annual cash retainer within five years of joining the Board, or as soon thereafter as practicable. Deferred stock units held in the Merck common stock account under the Directors’ Deferral Plan are counted toward the target goal. Any Director may request that the Governance Committee consider whether the target ownership level is appropriate in view of such Director’s personal circumstances.
As of December 31, 2022,2023, all Directors serving at least three years have either met or exceeded these stock ownership requirements. Dr. Mayo joined the Board effective March 15, 2021 and Mr. Baker joined the Board effective May 24, 2022 and, each is making progress toward meetingas of the date hereof, has met his stock ownership guidelines.requirements.
Merck & Co., Inc. 20232024 Proxy Statement
|
÷ ÷ ÷ | 43 |
20222023 Director Compensation
The table below summarizes the annual compensation for our non-employee Directors for the fiscal year ended December 31, 2022.2023.
Mr. Davis and Mr. Frazier areis the only DirectorsDirector who were officerswas an officer and employeesemployee of the Company during 2022,2023, and theyhe did not receive any additional compensation for theirhis Board service in 2022.2023.
| Director Compensation for Fiscal Year Ended December 31, 2022 | Director Compensation for Fiscal Year Ended December 31, 2023 | |||||||||||||||||||||||
Name | Fees Earned or Paid in Cash ($) | All Other Compensation ($)(2) | Total ($) | Fees Earned or Paid in Cash ($) | All Other Compensation ($)(1) | Total ($) | ||||||||||||||||||
Douglas M. Baker, Jr.(1) |
| $72,527 |
|
| $220,000 |
| $ | 292,527 |
| |||||||||||||||
Douglas M. Baker, Jr. | ||||||||||||||||||||||||
Douglas M. Baker, Jr. | ||||||||||||||||||||||||
Douglas M. Baker, Jr. | ||||||||||||||||||||||||
Douglas M. Baker, Jr. | $127,639 | $220,000 | $347,639 | |||||||||||||||||||||
Mary Ellen Coe | ||||||||||||||||||||||||
Mary Ellen Coe | ||||||||||||||||||||||||
Mary Ellen Coe | ||||||||||||||||||||||||
Mary Ellen Coe |
| 130,000 |
|
| 220,000 |
|
| 350,000 |
| 122,361 | 220,000 | 342,361 | ||||||||||||
Pamela J. Craig |
| 150,000 |
|
| 222,500 |
|
| 372,500 |
| |||||||||||||||
Pamela J. Craig | ||||||||||||||||||||||||
Pamela J. Craig | ||||||||||||||||||||||||
Pamela J. Craig | 150,000 | 250,000 | 400,000 | |||||||||||||||||||||
Thomas H. Glocer | ||||||||||||||||||||||||
Thomas H. Glocer | ||||||||||||||||||||||||
Thomas H. Glocer | ||||||||||||||||||||||||
Thomas H. Glocer |
| 180,000 |
|
| 220,000 |
|
| 400,000 |
| 180,000 | 250,100 | 430,100 | ||||||||||||
Risa J. Lavizzo-Mourey, M.D. |
| 120,000 |
|
| 233,500 |
|
| 353,500 |
| |||||||||||||||
Risa J. Lavizzo-Mourey, M.D. | ||||||||||||||||||||||||
Risa J. Lavizzo-Mourey, M.D. | ||||||||||||||||||||||||
Risa J. Lavizzo-Mourey, M.D. | 120,000 | 245,000 | 365,000 | |||||||||||||||||||||
Stephen L. Mayo, Ph.D. | ||||||||||||||||||||||||
Stephen L. Mayo, Ph.D. | ||||||||||||||||||||||||
Stephen L. Mayo, Ph.D. | ||||||||||||||||||||||||
Stephen L. Mayo, Ph.D. |
| 130,000 |
|
| 220,000 |
|
| 350,000 |
| 130,000 | 220,000 | 350,000 | ||||||||||||
Paul B. Rothman, M.D. |
| 150,000 |
|
| 275,000 |
|
| 425,000 |
| |||||||||||||||
Paul B. Rothman, M.D. | ||||||||||||||||||||||||
Paul B. Rothman, M.D. | ||||||||||||||||||||||||
Paul B. Rothman, M.D. | 150,000 | 230,000 | 380,000 | |||||||||||||||||||||
Patricia F. Russo | ||||||||||||||||||||||||
Patricia F. Russo | ||||||||||||||||||||||||
Patricia F. Russo | ||||||||||||||||||||||||
Patricia F. Russo |
| 140,000 |
|
| 220,000 |
|
| 360,000 |
| 140,000 | 220,000 | 360,000 | ||||||||||||
Christine E. Seidman, M.D. |
| 130,000 |
|
| 247,000 |
|
| 377,000 |
| |||||||||||||||
Christine E. Seidman, M.D. | ||||||||||||||||||||||||
Christine E. Seidman, M.D. | ||||||||||||||||||||||||
Christine E. Seidman, M.D. | 130,000 | 220,000 | 350,000 | |||||||||||||||||||||
Inge G. Thulin | ||||||||||||||||||||||||
Inge G. Thulin | ||||||||||||||||||||||||
Inge G. Thulin | ||||||||||||||||||||||||
Inge G. Thulin |
| 120,000 |
|
| 220,000 |
|
| 340,000 |
| 120,000 | 220,000 | 340,000 | ||||||||||||
Kathy J. Warden |
| 130,000 |
|
| 220,000 |
|
| 350,000 |
| |||||||||||||||
Kathy J. Warden | ||||||||||||||||||||||||
Kathy J. Warden | ||||||||||||||||||||||||
Kathy J. Warden | 130,000 | 220,000 | 350,000 | |||||||||||||||||||||
Peter C. Wendell |
| 120,000 |
|
| 250,000 |
|
| 370,000 |
| |||||||||||||||
Peter C. Wendell(2) | ||||||||||||||||||||||||
Peter C. Wendell(2) | ||||||||||||||||||||||||
Peter C. Wendell(2) | ||||||||||||||||||||||||
Peter C. Wendell(2) | 120,000 | 250,000 | 370,000 | |||||||||||||||||||||
| (1) |
|
Represents credits in the form of cash-settled deferred stock units (phantom shares) of Merck common stock to the Directors’ Deferral Plan. |
Figures also include charitable contributions made by the Merck Foundation under its matching gift program on behalf of the following Directors: |
Director Name | Matched Charitable
| |||||
Craig |
| |||||
Glocer | 30,100 | * | ||||
Lavizzo-Mourey |
| |||||
|
| |||||
|
| |||||
Wendell | 30,000 | |||||
* Includes $25,000 of charitable contributions made by the Merck Foundation in | ||||
| (2) | Mr. Wendell is retiring from the Board effective as of |
Changes to Non-Employee Director Compensation Program effective 2024
The Governance Committee reviews the Company’s non-employee Director compensation program on a biennial basis. In 2023, the Governance Committee conducted such a review in consultation with FW Cook, the C&MD Committee’s independent compensation consultant.
The review included FW Cook’s analysis of both compensation levels and program design compared to Merck’s peer groups that are used for executive compensation competitive benchmarking—a U.S. pharmaceutical peer group and a supplemental peer group comprised of the Dow Jones Industrial Average companies, excluding financial services companies (as described on page 49). Based on this review and the recommendation of FW Cook, the Governance Committee submitted its findings to the full Board in November 2023 and recommended that the full Board approve changes to the non-employee Director compensation program to better align leadership position retainers with external peer companies prior to the Governance Committee’s biennial Director compensation program review in 2025. Based on the results of FW Cook’s analysis and the Governance Committee’s recommendation, the Board approved the following changes to the non-employee Director compensation program effective April 1, 2024:
Increased Lead Director retainer from $40,000 to $50,000; and
Increased Committee chair retainer from $20,000 to $25,000.
The Governance Committee will continue to conduct, on a biennial basis, a competitive assessment of our non-employee Director compensation program with the goal of maintaining it at or near the median of our external peer groups.
Merck & Co., Inc. 20232024 Proxy Statement
| 44 | | | | | |
| |||||||
Proposal 2 Non-Binding Advisory Vote to |
We are pleased to provide our shareholders the opportunity to vote on a non-binding, advisory resolution to approve the compensation of our Named Executive Officers as disclosed in this proxy statement, including the Compensation Discussion and Analysis, compensation tables and the narrative discussion accompanying the tables, beginning on page 43.45. As described in the CD&A, our executive compensation programs are principally designed to reward executives based on the achievement of Company and individual performance objectives which, as a whole, are intended to drive sustainable long-term value creation for shareholders and reflect and maintain our position as an industry leader in the development of innovative medicines. The compensation of our NEOs is also designed to enable us to attract, engage and retain talented, high-performing and experienced executives in a competitive market.
In order to align executive pay with operational performance and the creation of long-term shareholder value, a significant portion of compensation paid to our NEOs is allocated to annual cash incentives and long-term equity incentives, which are both directly linked to Company and/or stock price performance. For 2022,2023, approximately 90%91% and 81%82%, respectively, of the CEO’s and other NEOs’ annual target total direct compensation was variable based on our operating performance and/or our stock price.
In addition, management and the C&MD Committee continually review the compensation programs for the NEOs to ensure they achieve the desired goals of reinforcing alignment of officer incentives with the interests of shareholders and linking compensation to performance as measured by operational results. As a result, we have adopted the policies and practices described on page 4648 to further align pay with operational performance and increases in long-term shareholder value while minimizing incentives that could lead to excessive risk-taking.
We are asking shareholders to indicate their support for the NEO compensation as described in this proxy statement. Accordingly, the following resolution will be submitted for approval by shareholders at the 20232024 Annual Meeting:
“Resolved, that the compensation paid to the Company’s Named Executive Officers, as disclosed pursuant to Item 402 of Regulation S-K, including the Compensation Discussion and Analysis, compensation tables and the narrative discussion described in pages 43-8545-87 of this proxy statement, is hereby APPROVED on an advisory basis.”
The shareholder vote on this resolution will not be binding on management, the C&MD Committee or the Board and will not be construed as overruling any decision by management, the C&MD Committee or the Board. However, the Board and the C&MD Committee value the opinions of our shareholders as expressed through their votes and other communications. In 2022,2023, shareholders continued their support of our executive compensation programs with approximately 92%91% of the votes cast for approval of a similar proposal. We will continue to give careful consideration to the outcome of the advisory vote on executive compensation and to the opinions of our shareholders when making compensation decisions.
At our 20172023 Annual Meeting of Shareholders, our shareholders voted in support of annual advisory votes on future executive compensation proposals. In Proposal 3, we are asking our shareholders to recommend, on an advisory basis, how frequently we should provide future advisory “say on pay” votes. The Board of Directors has adopted a policy providing for annual “say on pay” advisory votes. The Board expects that the next “say on pay” vote will occur in 2024.2025.
FOR
The Board of Directors recommends that shareholders vote FOR the resolution to approve, on an advisory basis, the compensation of our Named Executive Officers.
|
Merck & Co., Inc. 20232024 Proxy Statement
Compensation Discussion and Analysis | | | | | |
45 |
| |||||||
|
This CD&A describes the material elements of compensation for our 20222023 Named Executive Officers.
Named Executive Officers
Robert M. Davis
Chairman, Chief Executive Officer and President |
|
Caroline Litchfield
Executive Vice President and Chief Financial Officer |
President, Merck Manufacturing Division |
Richard R. DeLuca, Jr. Executive Vice President and President, Merck Animal Health |
Dean Li, M.D.,
Executive Vice President and President, Merck Research Laboratories |
|
Table of Contents
| 47 | |||||||
| 47 | |||||||
| 47 | |||||||
| 48 | |||||||
| 48 | |||||||
| 49 | |||||||
| 60 | |||||||
Merck & Co., Inc.2023 2024 Proxy Statement
| 46 |
ç ç ç |
Compensation Discussion and Analysis Executive Summary |
Executive Summary
2022In 2023, our Company continued to make progress on developing and delivering transformative therapies and vaccines to help save and improve lives around the world. We achieved strong operational performance and made significant advancements in our pipeline, including with strategic acquisitions, collaborations, and partnerships to provide patients the next generation of innovations.
The excellence of our commercial and operational execution enabled us to deliver 12% sales growth (excluding the impact of foreign exchange and sales of LAGEVRIO). As a result, we exceeded the Revenue and Pre-Tax Income targets in our 2023 Scorecard. Our commercial success was andriven by robust performance across key areas, particularly Oncology, Vaccines, and Animal Health. KEYTRUDA experienced exceptional yeargrowth of 21% (excluding foreign exchange), reaching over $25 billion in sales, driven by increased uptake in earlier-stage cancers and continued strong global need of patients with metastatic disease. Our vaccines portfolio also showed strong growth, with GARDASIL/GARDASIL 9 sales growing by 33% (excluding foreign exchange), due to strong global demand, particularly in China. Our continued launch of VAXNEUVANCE, for Merck, reflectingthe prevention of pediatric pneumococcal disease, generated $665 million in sales. Our Animal Health business achieved solid growth of 3% (excluding foreign exchange), driven equally by Companion Animal and Livestock product segments.
In addition to commercial success, our Company made significant progress in our ability to impact livespipeline. We initiated over 20 phase 3 studies across eight novel assets and received over 25 regulatory approvals in major markets around the world, throughhighlighting the breadth and depth of our innovativepipeline. Examples of our progress in 2023 include the FDA’s grant of priority review for sotatercept, our Company’s novel investigational activin signaling inhibitor, for the treatment of adult patients with pulmonary arterial hypertension, the FDA’s grant of priority review for our new Biologics License Application for V116, our Company’s investigational 21-valent pneumococcal conjugate vaccine specifically designed to help prevent invasive pneumococcal disease and pneumococcal pneumonia in adults, and the FDA’s approval of an additional indication for WELIREG in patients with advanced renal cell carcinoma, making it the first novel therapeutic class available for this population since 2015.
Our pipeline advancements were complemented by strategic business development activities, including the acquisitions of Prometheus Biosciences, Inc. and Imago BioSciences, Inc., and a collaboration with Daiichi Sankyo. These initiatives expanded our Company’s capabilities and potential reach in the fields of autoimmune conditions, hematology, and antibody-drug conjugates across multiple types of cancer.
Furthermore, we are proud of our ability to have reached more than 500 million people in 2023 with our medicines and keepingvaccines. Our science-led strategy, which keeps patients at the center of everything we do. We achieved strong operational performancedo, helps drive long-term value creation. As previewed in last year’s proxy and advancedsummarized below, we increased our broad pipeline, with important successes for our existing assets complemented by new assets acquired through a portfolio of strategic acquisitions, collaborations, and partnerships. These accomplishments enabled us to deliver meaningful results in 2022 and have positioned us to generate long-term innovation and value for both patients and shareholders.
Our efforts and underlying business strength enabled us to deliver 22% sales growth (26% excluding the impact of foreign exchange or “ex-exchange”). As a result, we exceeded our Revenue and Pre-Tax Income targets for our 2022 Scorecard. Commercially, we achieved very strong results across our key growth drivers, including KEYTRUDA, GARDASIL / GARDASIL 9, and Animal Health. KEYTRUDA grew 27% (ex-exchange) to just under $21 billion, driven by strong global demand for in-line indications, as well as continued global expansion from new approvals. In the U.S., KEYTRUDA grew across all key tumor types and continues to benefit from an uptake in earlier stage cancers including triple negative breast cancer, as well as certain types of renal cell carcinoma and melanoma. Overall, our vaccines portfolio experienced strong demand, particularly in major markets outside the U.S. and benefited from 27% (ex-exchange) growth of GARDASIL / GARDASIL 9 and the pediatric launch of VAXNEUVANCE, for the prevention of pneumococcal disease. Our Animal Health business grew by 6% (ex-exchange) and is well positioned for continued success.
We continued to strengthen our pipeline with significant advancements across oncology, vaccines, infectious diseases, and cardiology. We remain committed to transforming the landscape of cancer therapy with an ongoing focus on treating earlier stagesdriving sustainable business outcomes by linking the compensation of the disease and further improving patient outcomes across tumor types. In collaboration with Moderna, we announced positive Phase 2 results for V940/mRNA-4157, in combination with Keytruda,most employees, including our executives, to treat stage III and IV melanoma. This investigational neoantigen therapy uses mRNA technology with KEYTRUDAsustainability metrics focused on driving greater access to target the unique mutational signature of an individual patient’s tumor.
We also executed more than 90 transactionshealth to augment our pipeline, including strategic acquisitions, collaborations, and partnerships. These transactions spanned all phases of research and development, from discovery to Phase 3 assets. Some examples include our collaboration with Orion Corporation for the development and commercialization of a treatment for metastatic castration-resistant prostate cancer; our acquisition of Imago BioSciences, Inc. to expand our pipeline in the field of hematology; our global license and collaboration with Kelun-Biotech to develop up to seven investigational antibody-drug conjugate candidates for the treatment of cancer; and our collaboration with Orna Therapeutics to discover, develop, and commercialize multiple programs, including vaccines and therapeutics in the areas of infectious disease and oncology, using Orna’s engineered circular RNA technology.
Finally, when it comes to how Merck impacts the lives of peoplepatients around the world and on the American Cancer Society recently published data from women aged 20-24, who received the HPV vaccine, showing a 65% reductionengagement and inclusion of employees.
In summary, our success in cervical cancer from 2012 through 2019*. We are encouraged2023 was driven by the role GARDASIL / GARDASIL 9 playsexceptional operational execution, robust commercial growth, significant advancements in helpingour pipeline, and strategic business development. These achievements position our Company for continued success in discovering and delivering innovative therapies and vaccines to prevent certain HPV related cancers.
Our strong performance in 2022 drove above-target payouts for our annual incentive plansave and our 2020-2022 PSU awards as illustrated in the tables below.improve lives.
|
Scorecard Performance |
Financial Performance | ||||||||||||||||||||
Target($B) | Actual($B) | Weighting | Score | |||||||||||||||||
Revenue |
| $56.81 |
|
| $60.30 |
|
| 40 | % |
| 200 | % | ||||||||
Pre-Tax Income |
| $21.05 |
|
| $22.95 |
|
| 40 | % |
| 176 | % | ||||||||
Non-Financial Performance | ||||||||||||||||||||
Pipeline |
| 20 | % |
| 141 | % | ||||||||||||||
Total Payout |
|
| 178 | % | ||||||||||||||||
Financial Performance | ||||||||||||||||||||
Target($B) | Actual($B) | Weighting | Score | |||||||||||||||||
Revenue |
| $57.94 |
|
| $60.29 |
|
| 35 | % |
| 158 | % | ||||||||
Pre-Tax Income |
| $21.20 |
|
| $22.52 |
|
| 35 | % |
| 152 | % | ||||||||
Non-Financial Performance | ||||||||||||||||||||
Pipeline |
| 20 | % |
| 147 | % | ||||||||||||||
Sustainability |
| 10 | % |
| 100 | % | ||||||||||||||
Total Payout |
|
| 148 | % | ||||||||||||||||
PSU Performance |
| Target | Actual | Weighting | Score | |||||||||||||||||
1-Year EPS(2) |
| $5.69 |
|
| $5.62 |
|
| 33% |
|
| 90% |
| ||||||||
| Peer Median | Merck | Weighting | Score | |||||||||||||||||
3-Year R-TSR(2) |
| 10.40% |
|
| 14.27% |
|
| 67% |
|
| 119% |
| ||||||||
Total Payout |
|
| 110% |
| ||||||||||||||||
| Target | Actual | Weighting | Score | |||||||||||||||||
1-Year EPS(2) |
| $6.58 |
|
| $7.17 |
|
| 33% |
|
| 175% |
| ||||||||
| Peer Median | Merck | Weighting | Score | |||||||||||||||||
3-Year R-TSR(2) |
| 3.70% |
|
| 14.60% |
|
| 67% |
|
| 154% |
| ||||||||
Total Payout |
|
| 161% |
| ||||||||||||||||
| (1) |
|
| (2) |
|
Merck & Co., Inc. 20232024 Proxy Statement
Compensation Discussion and Analysis Executive Compensation Overview |
÷ ÷ ÷ | 47 |
Executive Compensation Overview
Our Industry Environment
The pharmaceutical industry is science-focused and requires experimentation and long-term commitment of financial resources to foster innovation. The Company is at the forefront of research to deliver innovative health solutions that advance the prevention and treatment of diseases in people and animals. Ultimately, our work has the potential to have an enormous impact on global health and well-being. Because of the inherent complexity and dynamic science of human and animal health, even with flawless execution, we risk failure. In addition:
The costs associated with innovation are increasing while relative return is decreasing due to ongoing pricing pressure.
The number of products available to treat or prevent a particular disease or condition typically increases over time, which can limit the commercial potential of key products.
It generallyGenerally, it takes 10 to 15 years to discover, develop, and bring a new productmedicine or vaccine to market.
Competition for qualified talent in the pharmaceutical industry, both in the U.S. and internationally, is intense.
Our Executive Compensation Strategy
We strive to balance the need to deliver market-competitive pay within a framework that provides the appropriate mix of fixed and variable, at-risk compensation to attract, retain, and motivate talent and align with our pay-for-performance objectives.
Our executive compensation program is designed to… | |||||
 | Support our efforts to attract and retain the brightest and most innovative minds in business, research, and academia. | ||||
| Align the interests of our executives with the interests of our shareholders to ensure prudent actions that will generate long-term value. | ||||
 | Reward our executives based on the achievement of sustained financial and operational performance and demonstrated leadership. | ||||
| Support a shared, one-company mindset of performance and accountability to deliver on business objectives.
| ||||
Merck & Co., Inc. 20232024 Proxy Statement
| 48 |
ç ç ç |
Compensation Discussion and Analysis Executive Compensation Overview |
Compensation Policies and Practices
Our executive compensation and corporate governance programs are designed to closely link pay with operational performance and increases in long-term shareholder value while minimizing incentives that could lead to excessive risk-taking. To help us accomplish these important objectives, we have adopted the following policies and practices:
We do… | We do not… | ||||||
| Utilize a relative total shareholder return metric in the PSU program to align the payout with long-term stock performance and shareholder experience
|
| |||||
|
| ||||||
| Monitor LTI program share utilization regularly relative to both industry standards and versus our
| ||||||
|
Conduct annual competitive benchmarking to ensure executive officer compensation is aligned to market
| ||||||
|
| ||||||
|
Include caps on annual cash incentive and PSU program payouts and thresholds below which no payouts are earned
| ||||||
| Offer limited perquisites that are supported by business interests | ||||||
|
Retain an independent compensation consultant that reports directly to the C&MD Committee
| ||||||
|
Maintain robust stock ownership requirements and share retention policies
| ||||||
|
Maintain that exceeds the NYSE listing requirements
| ||||||
|
Conduct assessments to identify and mitigate risk in our compensation
| ||||||
| Provide dividend equivalents only on earned Restricted Stock Units (“RSUs”) and PSUs | ||||||
|
Require double-trigger vesting of equity in the event of a change in control (i.e., there must be both a change in control and an involuntary termination)
| ||||||
|
Avoid employment agreements
| ||||||
Say-on-Pay Advisory Vote
In | 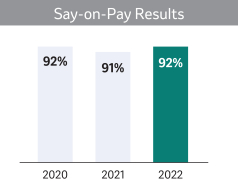 .. .. |
We ask that our shareholders approve, on an advisory basis, the compensation of our NEOs as further described in Proposal 2 on page 42.44.
Merck & Co., Inc. 20232024 Proxy Statement
Compensation Discussion and Analysis Peer Groups |
÷ ÷ ÷ | 49 |
Peer Groups
Merck’s Primary Peer Group
Individual executive officer compensation levels and opportunities are compared to a peer group of large multinational pharmaceutical companies approved by the C&MD Committee that participate in a pharmaceutical industry compensation survey conducted by Willis Towers Watson, an independent consulting firm. In setting compensation levels for 2022,2023, the C&MD Committee reviewed the survey results for the following peer companies that Merck competes with to attract talented, high-performing executives.executives (the “primary peer group”). The C&MD Committee occasionallyregularly reviews segmented information focused on the companies in the primary peer group that are headquartered in the U.S. because practices outside the U.S. can differ geographically.
Primary Peer Group Companies
AbbVie Amgen AstraZeneca Bristol-Myers Squibb Eli Lilly Gilead Sciences GlaxoSmithKline Johnson & Johnson Novartis Pfizer Roche Holding AG Sanofi
All numbers are as of 12/31/
| 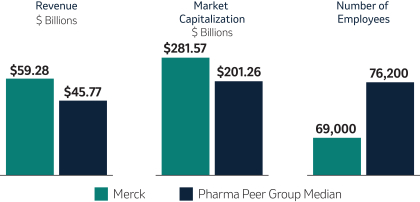 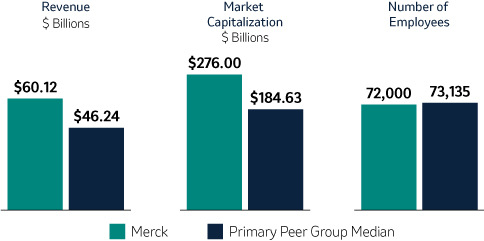 |
In 2021, the C&MD Committee approved the addition of Gilead Sciences, Inc. to our primary peer group, effective January 1, 2022.
Merck’s Supplemental Peer Group
In addition to the pharmaceuticalprimary peer group described above, we also use a supplemental peer group consisting of the companies that comprise the Dow Jones Industrial Average (excluding the financial services companies) as a secondary reference for CEO compensation and for other compensation-related practices (for example, share usage and dilution, change in control policy design, and stock ownership and retention guidelines). Merck is a member of the Dow Jones Industrial Average, and we believe this group provides insight into practices among companies of similar scale and complexity that operate across a variety of industries, providing us with a broader view of market pay, policies, and practices.
Supplemental Peer Group Companies(1)
|
| |||
3M Amgen Apple Boeing Caterpillar Chevron Cisco Systems Coca-Cola Dow Home Depot Honeywell IBM Intel | Johnson & Johnson McDonald’s Microsoft Nike Procter & Gamble salesforce.com UnitedHealth Group Verizon Visa Walgreens Walmart Walt Disney | |||
(1) Reflects Dow Jones Industrial Average companies (excluding the financial services companies) as of the beginning of
All numbers are as of 12/31/
| ||||
Our overarching strategy is to position our executives’ target TDC at the median, on average, with variability by individual executive based on scope and complexity of role, market availability of proven talent, experience, leadership, sustained performance over time, potential for advancement as part of succession planning, and other unique factors that may exist from time to time. This median target compensation philosophy ensures that actual realized compensation varies above or below market levels based on attainment of longer-term goals and changes in shareholder value, and overall costs and share dilution are reasonable and sustainable relative to market practices.
Merck & Co., Inc. 20232024 Proxy Statement
| 50 |
ç ç ç |
Compensation Discussion and Analysis Detailed Discussion and Analysis |
Detailed Discussion and Analysis
Additional information regarding our 20222023 Named Executive Officers and the material elements of their compensation, as reported in the Summary Compensation Table on page 62,63, is described below*.
|
|
Compensation Decisions for
| ||||||||
Robert M. Davis Chairman, Chief Executive Officer and President
|
• Increased base salary by • Maintained annual incentive target percentage • Increased LTI target by • Changes resulted in |
| ||||||||
Age:
| ||||||||||
Tenure*:
| ||||||||||
| |||||||||
|
|
| |||||||
| |||||||||
| |||||||||
|
|
|
| ||||||||
Merck & Co., Inc. 2023 Proxy Statement
|
|
|
|
Compensation Decisions for
| |||||||
Caroline Litchfield Executive Vice President and Chief Financial Officer
|
• Increased base salary by • Maintained annual incentive target percentage • Increased LTI target by • Changes resulted in |
| |||||||
Age:
| |||||||||
Tenure*:
| |||||||||
* Compensation shown for each executive is rounded to the nearest dollar and length of tenure is rounded to the nearest year.
Merck & Co., Inc. 2024 Proxy Statement
Compensation Discussion and Analysis Detailed Discussion and Analysis | ÷ ÷ ÷ ÷ | 51 |
|
|
Compensation Decisions for
| ||||||||
Executive Vice President and President, Merck Manufacturing Division
|
• Set base salary at • Set annual incentive target at • |
| ||||||||
Age:
| ||||||||||
Tenure*:
| ||||||||||
|
Merck & Co., Inc. 2023 Proxy Statement
|
|
|
|
Compensation Decisions for | |||||||
Richard R. DeLuca, Jr. Executive Vice President and President, Merck Animal Health | • Increased base salary by $75,000 • Maintained annual incentive target percentage • Increased LTI target by $200,000 • Changes resulted in a 7.4% increase in target TDC |
| |||||||
Age: 61 | |||||||||
Tenure*: 12 Years | |||||||||
Merck & Co., Inc. 2024 Proxy Statement
| 52 | ç ç ç ç | Compensation Discussion and Analysis Detailed Discussion and Analysis |
Compensation Decisions for 2023
| ||||||||
Dean Li, M.D., Ph.D. Executive Vice President and President, Merck Research Laboratories
|
• Increased base salary by • Maintained annual incentive target percentage • Increased LTI target by • Changes resulted in |
| ||||||
Age:
| ||||||||
Tenure*:
| ||||||||
| ||||||||
| ||||||||
|
|
| ||||||
| ||||||||
| ||||||||
Merck & Co., Inc. 20232024 Proxy Statement
Compensation Discussion and Analysis The Elements of |
÷ ÷ ÷ | 53 |
The Elements of 20222023 Compensation
How Our Compensation Program Works
What We Reward | How We Link Pay To Performance | How We Pay | ||||||||||||||||
• Top and bottom-line performance that meets or exceeds the Board approved annual and long-term operating plans
• Pipeline accomplishments that advance our position as an industry-leading biopharmaceutical company • Achievement of strategic sustainability priorities that focus on greater access to health and on the engagement and inclusion of employees
• Decision-making that yields long-term value creation for shareholders
• Executing on our growth strategy by consistently seeking opportunities that complement or supplement our broad portfolio in key areas including Oncology, Vaccines, Cardiometabolic, and Animal Health
| • Inclusion of key financial and non-financial metrics in our annual cash incentive plan to ensure executives are rewarded for top and bottom-line performance,
• Long-term incentives comprised of a mix of performance share units and stock options, linking a substantial amount of pay opportunity to long-term company performance and increased shareholder value
• Majority of total target pay opportunity is at-risk and tied to company performance and/or long-term stock value
| • Overall target total pay opportunity, as well as each pay element, is assessed for competitiveness relative to primary and/or supplemental peer groups, which include similarly-sized pharmaceutical peers and Dow Jones Industrial Average companies, excluding financial services companies
• Competitive positioning is targeted to median of market; actual positioning varies based on a variety of factors, including scope and complexity of role, years of experience, demonstrated performance over time, and other factors
| ||||||||||||||||
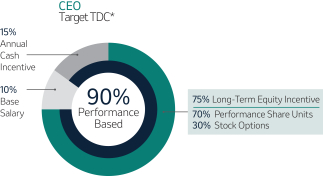
| 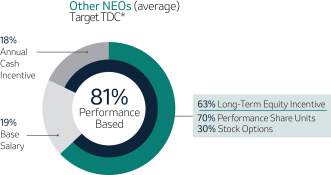 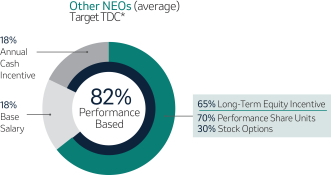 |
*Rounded based on full year long-term incentive and annual incentive targets (excluding one-time special awards).
TheEach year, the C&MD Committee recommends, and the independent members of the Board of Directors approve, the compensation for our CEO and, as applicable, our Executive Chairman.CEO. The C&MD Committee reviews and approves compensation for all other NEOs each year based on a variety of factors, including scope and complexity of role, experience, sustained leadership, and performance and competitive positioning as compared to our pharmaceuticalprimary and supplemental peer groups as described in more detail on page 47.49.
Additional details regarding the roles and responsibilities of the C&MD Committee are provided on page 15.
Merck & Co., Inc. 20232024 Proxy Statement
| 54 |
ç ç ç |
Compensation Discussion and Analysis The Elements of |
Base Salary
The C&MD Committee must balance
The table shows adjustments made to | Named Executive Officer | Annual Base Salary Increase % | Market/Promotional Adjustment % | New Base Salary | ||||||||||||||||||
Davis |
| 3.0 | % |
| No change |
|
| $1,545,000 |
| |||||||||||||
Frazier(1) |
| No change |
|
| No change |
|
| 1,250,000 |
| |||||||||||||
Litchfield |
| 3.0 |
|
| 5.3 | % |
| 975,000 |
| |||||||||||||
Guindo(2) |
| — |
|
| — |
|
| 700,000 |
| |||||||||||||
Li(3) |
| 3.0 |
|
| 28.6 |
|
| 1,250,000 |
| |||||||||||||
Zachary |
| 3.0 |
|
| No change |
|
| 974,702 |
| |||||||||||||
(1) Mr. Frazier retired from his position, effective November 30, 2022, and did not receive a salary increase or market adjustment during 2022. (2) Mr. Guindo was hired on July 1, 2022. (3) Dr. Li received an annual base salary increase of 3% and two market adjustments totaling 28.6%. |
| |||||||||||||||||||||
Annual Cash Incentive
| ||||||||||||||||||||||
The NEOs participate in the Executive
Award amounts under the EIP are | Named Executive Officer |
| 2021 Target Annual % of Base Salary(1) | 2022 Target Annual % of Base Salary(1) | ||||||||||||||||||
Davis |
| 150 | % |
| 150 | % | ||||||||||||||||
Frazier(2) |
| 100 |
|
| 100 |
| ||||||||||||||||
Litchfield |
| 100 |
|
| 100 |
| ||||||||||||||||
Guindo(3) |
| — |
|
| 80 |
| ||||||||||||||||
Li |
| 100 |
|
| 100 |
| ||||||||||||||||
Zachary |
| 95 |
|
| 95 |
| ||||||||||||||||
(1) Reflects annual incentive targets as of December 31 of the applicable year. (2) Mr. Frazier retired from his position as Executive Chairman, effective November 30, 2022. Mr. Frazier’s actual 2022 annual incentive was prorated based on his retirement date. (3) Mr. Guindo was hired on July 1, 2022. Mr. Guindo was not an NEO in 2021 and his actual 2022 annual incentive was prorated based on his hire date.
|
| |||||||||||||||||||||
The C&MD Committee must balance the need to deliver a competitive level of base salary with ensuring the appropriate mix of fixed to variable compensation for each NEO.
The table shows adjustments made to base salaries in 2023. All annual base salary increases were based on Merck’s U.S. salary increase budget for all employees, including the NEOs. | Named Executive Officer | | Annual Base Salary Increase % |
| | Market Adjustment % | | New Base Salary(1) | ||||||||||
Davis |
| 4.5 | % |
| No change |
|
| $1,615,000 |
| |||||||||
Litchfield |
| 4.5 |
|
| 10.9 | % |
| 1,125,000 |
| |||||||||
Chattopadhyay |
| — | (2) |
| — | (2) |
| 941,806 |
| |||||||||
DeLuca |
| 4.5 | (2) |
| 4.3 | (2) |
| 925,000 |
| |||||||||
Li |
| 4.5 |
|
| 7.5 |
|
| 1,400,000 |
| |||||||||
(1) Reflects base salary as of December 31, 2023. (2) Mr. Chattopadhyay was not an NEO in 2022. Although Mr. DeLuca was not an NEO in 2022, he was an NEO in 2021. As such, pursuant to SEC rules, we have included his 2022 compensation information in the Summary Compensation table.
|
| |||||||||||||||||
Annual Cash Incentive
| ||||||||||||||||||
The NEOs participate in the Executive Incentive Plan (“EIP”).
Award amounts under the EIP are determined based upon achievement of Company performance measures as reflected by the Company Scorecard. The overall EIP award fund cannot exceed 200% of the aggregate total target incentive amount for all participants. The maximum award amount for each NEO for 2023, excluding the impact of the Company Scorecard, is listed in the Grants of Plan-Based Awards table on page 73. | Named Executive Officer | | 2022 Target Annual % of Base Salary(1) |
| | 2023 Target Annual % of Base Salary(1) |
| |||||||||||
Davis |
| 150 | % |
| 150 | % | ||||||||||||
Litchfield |
| 100 |
|
| 100 |
| ||||||||||||
Chattopadhyay |
| — | (2) |
| 100 |
| ||||||||||||
DeLuca |
| 100 | (2) |
| 100 |
| ||||||||||||
Li |
| 100 |
|
| 100 |
| ||||||||||||
(1) Reflects annual incentive targets as of December 31 of the applicable year. (2) Mr. Chattopadhyay was not an NEO in 2022. Although Mr. DeLuca was not an NEO in 2022, he was an NEO in 2021. As such, pursuant to SEC rules, we have included his 2022 compensation information in the Summary Compensation table.
|
| |||||||||||||||||
Merck & Co., Inc. 20232024 Proxy Statement
Compensation Discussion and Analysis The Elements of |
÷ ÷ ÷ | 55 |
2022 Merck2023 Company Scorecard
Our Company Scorecard helps translate our strategic priorities into operational terms that enable tracking and measurement of our progress and performance against annual operating goals and critically important long-term strategic drivers of sustainablelong-term value creation, including goals tied to our research and development pipeline — each of which is measured in the context of compliance, health, safety, and environmental outcomes. The Company Scorecard may be adjusted based on an evaluation of these outcomes, recognizing the importance they play in driving Merck’s values and a culture of integrity. For 2022,2023, no such adjustment was applied. We believe creating greater accountability for the sustainable delivery of business goals will help drive financial results and long-term shareholder value. As a result, the C&MD Committee approved a new measure in our 2023 Company Scorecard that links the compensation of most employees, including our executives, to certain Sustainability metrics. The new design reflects our focus on driving greater access to health to patients around the world and on the engagement and inclusion of our employees, each of which is a strategic priority. Revenue and Pre-Tax Income are equally weighted at 35% (previously 40%), each based on the C&MD Committee’s belief that they are the key financial measures of our success during the year. The Pipeline goals are collectively weighted at 20% and are designed to ensure that we are focused on internal and external early discovery opportunities, late-stage clinical development progression, and regulatory filings and approvals. Finally, our new Sustainability metrics are collectively weighted at 10%.
The target, threshold, and maximum Revenue and Pre-Tax Income goals are set in relation to the Board-approved annual operating plan and the expectations of management. Each year, the Pipeline goals are recommended by the President of Merck Research Laboratories, reviewed by the Research Committee, and approved by the C&MD Committee. The Sustainability metrics are recommended by Merck’s Global Market Access, ESG, and Human Resources teams and approved by the C&MD Committee. Failure to achieve threshold performance on any of the metrics would result in forfeiture of the entire opportunity for that metric. If the combined results of the threefour metrics do not total at least 50, there would be no payout. The overall results of the Company Scorecard are calibrated so individuals may receive between 50% and 200% of their target award opportunity established for the annual performance period. Adjustments are applied to Revenue and Pre-Tax Income results using a consistent framework of adjustments to our reported financial results for incentive program purposes approved by the C&MD Committee to accurately reflect the operating performance of our business. For further explanation of these adjustments, please refer to Appendix B on page 111.106. The 20222023 Company Scorecard results are summarized below.
20222023 Company Scorecard(1)
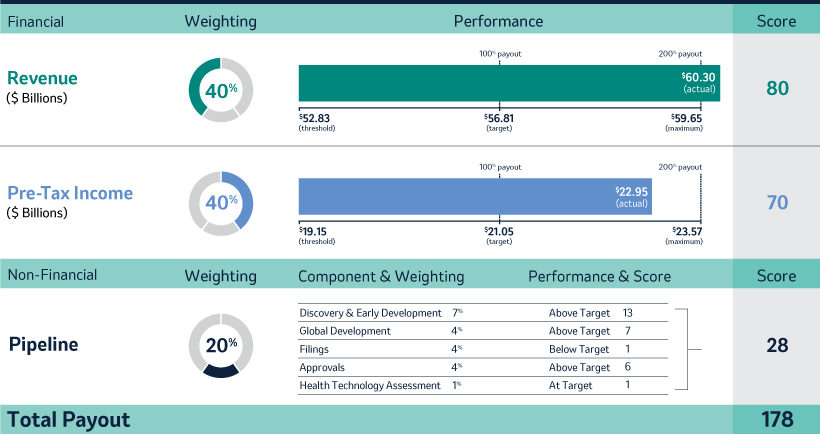
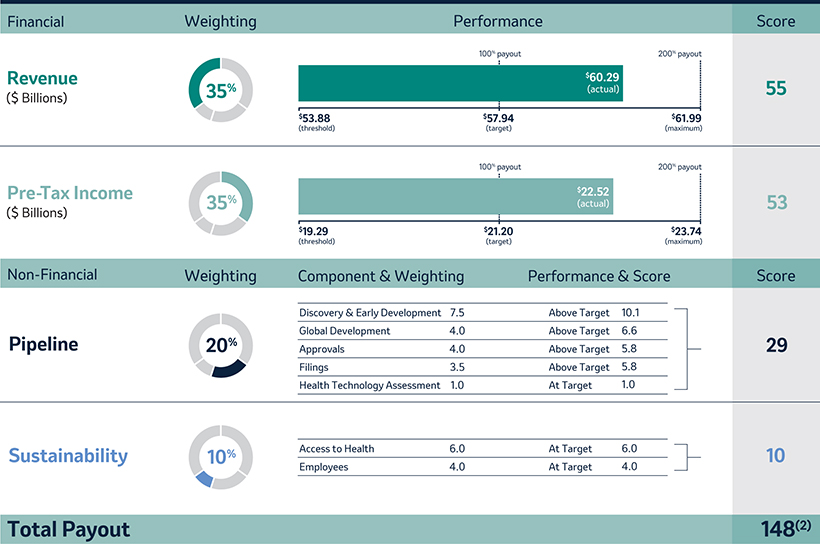
| (1) | Excluding the impact of variances in currency exchange rates versus budget and certain other items, consistent with plan |
Revenue:
Reported revenue of $59.28B was adjusted to $60.30B to exclude the impact of currency exchange rates (versus currency exchange rates budgeted in the annual operating plan). We exceeded our internal Revenue target of $56.81B due to strong performance in key pillars, including oncology and vaccines, as well as the significant contribution of LAGEVRIO.
| (2) | Rounded to the nearest whole number. |
Merck & Co., Inc. 20232024 Proxy Statement
| 56 |
ç ç ç |
Compensation Discussion and Analysis The Elements of |
Pre-Tax Income:Revenue:
Reported Pre-Tax Incomerevenue of $22.16B$60.12B was adjusted to $22.95B$60.29B to exclude the impact of currency exchange rates (versus currency exchange rates budgeted in the annual operating plan) and the associated impact of hyper-inflation in certain markets (consistent with plan design).
Pre-Tax Income:
Non-GAAP pre-tax income of $5.99B was adjusted to $22.52B to exclude the effect of certain business development transactions (consistentas well as the impact of currency exchange rates (versus currency exchange rates budgeted in the annual operating plan) and the associated impact of hyper-inflation in certain markets (all consistent with plan design and past practice)design). We exceeded our internal Pre-Tax income target of $21.05B due to the sales strength that was achieved coupled with our continued discipline in expense management.
Named Executive Officer 20222023 Annual Incentive Payouts
The table below shows the 20222023 annual cash incentives paid to the NEOs. The “Final Award” for each NEO is reflected in the “Non-Equity Incentive Plan Compensation” column of the Summary Compensation table.

| Target | ||||||||||||||||||||
Named Executive Officer | Annual Base Salary (as of 12/31/22) ($) | Annual Incentive (%) | Annual Incentive ($) | Company Scorecard Result (%) | Final Award ($) | |||||||||||||||
Davis |
| $1,545,000 |
|
| 150 | % |
| $2,317,500 |
|
| 178 | % |
| $4,125,150 |
| |||||
Frazier(1) |
| 1,250,000 |
|
| 100 |
|
| 1,143,836 |
|
| 178 |
|
| 2,036,028 |
| |||||
Litchfield |
| 975,000 |
|
| 100 |
|
| 975,000 |
|
| 178 |
|
| 1,735,500 |
| |||||
Guindo(2) |
| 700,000 |
|
| 80 |
|
| 282,301 |
|
| 178 |
|
| 502,496 |
| |||||
Li |
| 1,250,000 |
|
| 100 |
|
| 1,250,000 |
|
| 178 |
|
| 2,225,000 |
| |||||
Zachary |
| 974,702 |
|
| 95 |
|
| 925,967 |
|
| 178 |
|
| 1,648,221 |
| |||||
|
|
| Target | ||||||||||||||||||||
Named Executive Officer | Annual Base Salary (as of 12/31/23) ($) | Annual Incentive (%) | Annual Incentive ($) | Company Scorecard Result (%) | Final Award ($) | |||||||||||||||
Davis | $1,615,000 | 150 | % | $2,422,500 | 148 | % | $3,585,300 | |||||||||||||
Litchfield | 1,125,000 | 100 | 1,125,000 | 148 | 1,665,000 | |||||||||||||||
Chattopadhyay | 941,806 | 100 | 941,806 | 148 | 1,393,873 | |||||||||||||||
DeLuca | 925,000 | 100 | 925,000 | 148 | 1,369,000 | |||||||||||||||
Li | 1,400,000 | 100 | 1,400,000 | 148 | 2,072,000 | |||||||||||||||
Long-Term Equity Incentives
The long-term incentive program, consisting of a mix of PSUs and stock options, provides our NEOs with the opportunity to own Merck common stock, directly linking a substantial portion of their compensation to the returns realized by our shareholders. We use these two vehicles to ensure that our LTI program remains balanced, sustainable, and supportive of its objectives over a multi-year period.
20222023 Equity Award Mix
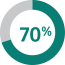 | Performance Share Units | |
| PSUs link realized compensation value to the achievement of critical financial objectives and align executives’ interests with those of our shareholders. The earned award varies based on results versus pre-determined performance goals, as well as long-term returns to shareholders as measured by relative stock price performance and dividend yield. | ||
| Stock Options Stock options align our executives’ interests with the interests of our shareholders because options only have financial value to the recipient if the price of our common stock at the time of exercise exceeds the stock price on the date of grant. As a result, we believe stock option grants encourage executives to focus on behaviors and initiatives that support sustained long-term stock price appreciation, which benefits all shareholders. | |
Merck & Co., Inc. 2023 Proxy Statement
|
|
Current LTI Grant Practices
All grants to executive officers are made under the Merck & Co., Inc. 2019 Stock Incentive Plan and approved by the C&MD Committee and, in the case of our CEO, and former Executive Chairman, recommended by the C&MD Committee and approved by the independent members of the Board of Directors. Annual PSU grants (with a 3-yearthree-year performance period) are generally made on the last business day in March and annual stock option grants are made on the third business day following the announcement of
Merck & Co., Inc. 2024 Proxy Statement
Compensation Discussion and Analysis The Elements of 2023 Compensation | ÷ ÷ ÷ ÷ | 57 |
our first quarter earnings. We may also selectively grant PSUs, stock options, and RSUs to executive officers on the third business day following the announcement of quarterly earnings generally as part of a new hire sign-on or for retention purposes. These dates were chosen to ensure that grants are made shortly after we have released information about our financial performance to the public. However, the C&MD Committee reserves the right to change the date when grants are made, in view of its responsibility to consider all facts and circumstances to ensure that grants are consistent with our compensation philosophy and objectives.
Stock options are granted at no less than fair market value on a fixed date or date of a particular event, with all required approvals obtained in advance of or on the actual grant date. Fair market value is the closing price of a share of CompanyMerck common stock on the grant date. The re-pricing of stock options is not permitted under the Incentive Stock Plan without prior shareholder approval.
20222023 LTI Grant Values
The 20222023 annual LTI grant values for the CEO former Executive Chairman, and other NEOs, as compared to the prior year, are shown in the following table. The number of shares associated with each award is set forth in the Grants of Plan-Based Awards table on page 71.73. The LTI grant value for Mr. Davis was increased by the Board in consideration of his first full year as CEO and the LTI grant values for the other NEOs were increased by the C&MD Committee to strengthen histheir competitive position versus our pharmaceutical peer group. The LTI value for Mr. Frazier was decreased by the Board in consideration of his first full year as Executive Chairman. LTI values for Ms. Litchfield, Dr. Li, and Ms. Zachary were increased to strengthen their competitive market positioning versus our pharmaceuticalprimary peer group and to recognizereflect their impact.expected future contributions in creating sustained long-term shareholder value.
| Target Grant Value(1) | Increase in Target Grant Value | |||||||||||
Executive Officer | 2021 | 2022 | ||||||||||
Davis(2) |
| $9,200,000 |
|
| $11,750,000 |
|
| +$2,550,000 |
| |||
Frazier(3) |
| 10,750,000 |
|
| 5,000,000 |
|
| -5,750,000 |
| |||
Litchfield |
| 2,200,000 |
|
| 2,750,000 |
|
| +550,000 |
| |||
Guindo(4) |
| — |
|
| — |
|
| — |
| |||
Li |
| 3,000,000 |
|
| 3,900,000 |
|
| +900,000 |
| |||
Zachary |
| 2,700,000 |
|
| 3,000,000 |
|
| +300,000 |
| |||
| Target Grant Value(1)
| Increase in Target Grant Value | |||||||||||
Executive Officer |
2022 |
2023 | ||||||||||
Davis | $11,750,000 | $13,500,000 | +$1,750,000 | |||||||||
Litchfield | 2,750,000 | 4,250,000 | +1,500,000 | |||||||||
Chattopadhyay | — | (2) | 3,300,000 | — | ||||||||
DeLuca | 3,000,000 | (2) | 3,200,000 | +200,000 | ||||||||
Li | 3,900,000 | 5,600,000 | +1,700,000 | |||||||||
| (1) | Grant values shown above will be different from the values shown in the Summary Compensation and Grants of Plan-Based Awards tables based on the fair value on grant date in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718 (“FASB ASC Topic |
| (2) | Mr. |
|
|
Merck & Co., Inc. 2023 Proxy Statement
|
|
PSU Program
At the beginning of each year, we review the design of our PSU program to ensure that our metrics are focused on the long-term measures that are most applicable to driving value for the Company and its shareholders over a three-year performance period. Payouts under the PSU program are formulaic and, as such, the C&MD Committee does not consider individual performance or use discretion when determining final awards.
Financial targets applicable to the PSUs are established based on our three-year financial plan, which considers a variety of factors, including management, Board, and external expectations and aspirations of our long-term performance. The financial targets for our currently outstanding PSUs are EPS and R-TSR. R-TSR performance versus our primary peer group is measured at the end of the three-year period and compares Merck’s average annual TSR to the median TSR of our pharmaceuticalthat peer group. Each percentage point of outperformance or underperformance versus the median modifies the earned award up or down by 5five percentage points. In the event of underperformance by more than 10 percentage points, there will not be a payout on the R-TSR portion of the award. In the event of outperformance, the payout on the R-TSR portion of the award cannot exceed 200%. If R-TSRour annualized TSR is negative, the payout on this portion of the award cannot exceed 100%, even if our R-TSR outperforms the median of the peer group.
Merck & Co., Inc. 2024 Proxy Statement
| 58 | ç ç ç ç | Compensation Discussion and Analysis The Elements of 2023 Compensation |
The 20202021 PSU program concluded at the end of 20222023 and the payout is described on the following page.below. Due to the complexities associated with disentangling our Organon business from a multi-year financial plan, we adjusted the design for the 2020 and 2021 programsprogram as described below. Our Organon business was successfully spun off in June 2021. Following the completion of the spin-off, we reverted to a three-year cumulative EPS and R-TSR design forbeginning with the 2022 PSU program, with 50% tied to EPS and 50% tied to R-TSR.
Program Performance Period | Original Program Design | Program Design as a result of the Organon Spin-off | |||
|
|
| |||
2021-2023 | 50% 3-Year EPS 50% 3-Year R-TSR | 33% 1-Year (2021) EPS 67% 3-Year R-TSR | |||
| 2022-2024 2023-2025
| 50% 3-Year EPS
| Not Applicable | |||
| (1) | Alternative design was established on grant date and part of original grant terms. |
Merck & Co., Inc. 2023 Proxy Statement
|
|
Payouts Under the 2020–20222021–2023 PSU Program Performance Period
For grants issued in 2020,2021, 70% of each NEO’s annual target LTI was converted to PSUs based on the closing price of Merck stock on the date of grant. The number of PSUs ultimately earned is based on our performance against the pre-established EPS target and R-TSR performance. As a result of the spin-off of Organon, the original number of PSUs granted were adjusted to preserve the same intrinsic value as was in place immediately prior to the adjustments.
For the 2020-20222021-2023 performance period, as a result of the Organon spin-off, one-year (2020)(2021) EPS was weighted at 33%, and three-year R-TSR versus our pharmaceuticalprimary peer group was weighted at 67%. If the Organon spin-off did not occur, three-year cumulative EPS would have been used. The outcome of the combined performance resulted in an actual payout of 110%161% as illustrated below.
The 110%161% payout was based on our strong EPS and R-TSR performance (both above 150%) during the performance period due to the Emergency Use Authorization of LAGEVRIO in the United States in 2021 and our TSR outperforming the median of our pharmaceuticalprimary peer group by nearly 4%, resulting in a R-TSR payout of 119%11%. EPS results were below target, largely due to the negative impact of COVID-19 on our 2020 financial results.
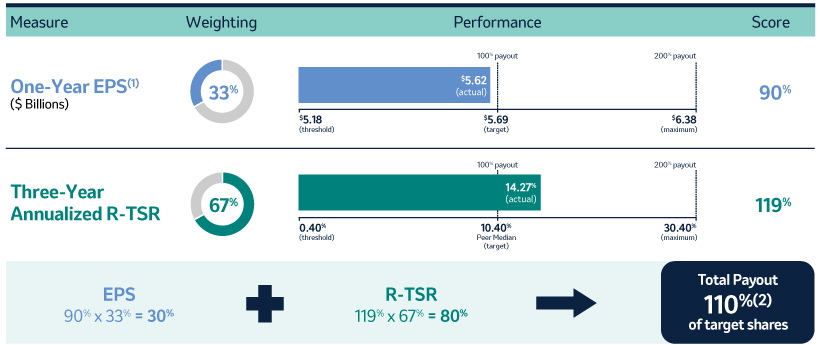
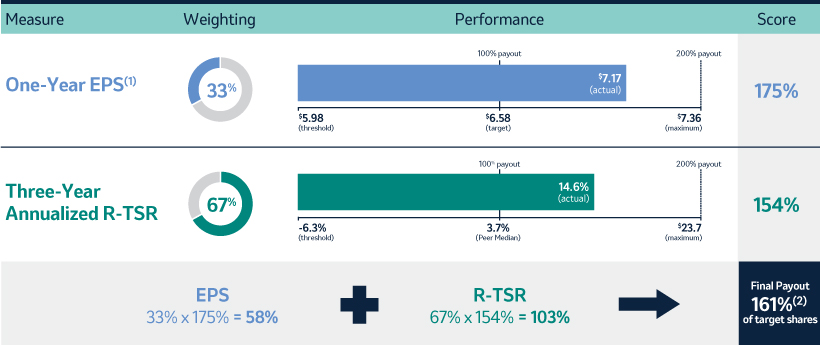
As a result of the Organon spin-off, the performance period for EPS was adjusted to one year, the weighting for EPS was reduced from 50% to 33%, and the weighting for R-TSR was increased from 50% to 67%. |
Rounded to the nearest whole percentage. |
Merck & Co., Inc. 20232024 Proxy Statement
Compensation Discussion and Analysis The Elements of | ÷ ÷ ÷ ÷ | 59 |
Named Executive Officer PSU Distribution
Based on the final payout of 110%161%, the NEOs received the following number of shares of Merck common stock, including dividends accrued during the performance period and paid in shares:
Named Executive Officer | Pre-Spin Target Award (# of shares) | Adjusted Post-Spin (# of shares) | Final Award(2) (# of shares) | Pre-Spin Target Award (# of shares) | Adjusted Post-Spin Target Award(1) (# of shares) | Final Award(2) (# of shares) | ||||||||||||||||||
Davis | 36,392 | 37,508 | 44,233 | |||||||||||||||||||||
Frazier | 143,293 | 147,689 | 174,168 | |||||||||||||||||||||
Davis | ||||||||||||||||||||||||
Davis | ||||||||||||||||||||||||
Davis | 83,539 | 86,102 | 148,374 | |||||||||||||||||||||
Litchfield | 3,392 | 3,496 | 4,123 | |||||||||||||||||||||
Litchfield | ||||||||||||||||||||||||
Litchfield | ||||||||||||||||||||||||
Litchfield | 19,977 | 20,590 | 35,481 | |||||||||||||||||||||
Guindo(3) | — | — | — | |||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||
Chattopadhyay | 25,425 | 26,205 | 45,157 | |||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||
DeLuca | 24,517 | 25,269 | 43,544 | |||||||||||||||||||||
Li | 4,159 | 4,287 | 5,056 | |||||||||||||||||||||
Zachary | 22,745 | 23,443 | 27,646 | |||||||||||||||||||||
Li | ||||||||||||||||||||||||
Li | ||||||||||||||||||||||||
Li | 27,241 | 28,077 | 48,384 | |||||||||||||||||||||
| (1) | As a result of the Organon spin-off, the original number of PSUs granted in |
| (2) | Includes accrued dividends distributed in shares following final award determination. |
|
Additional information regarding the payouts under the 2020-20222021-2023 PSU performance period is provided in the Option Exercises and Stock Vested table on page 76.77.
To accurately reflect the operating performance of our business, the C&MD Committee approved a consistent framework of adjustments to our reported financial results for incentive program purposes. For further explanation of these adjustments and our GAAP versus Non-GAAP results, please refer to Appendices A and B on pages 109104 and 111,106, respectively.
Other Employee Benefits
Similar to Merck’s other salaried, U.S.-based employees, the NEOs participate in a variety of retirement, health and welfare, and paid time-off benefits designed to enable us to attract and retain our workforce in a highly competitive market. Pension and savings plans help employees save and prepare financially for retirement. Health and welfare and paid time-off benefits help ensure that we have a healthy, productive, and focused workforce.
Additionally, senior management employees, including the NEOs, are provided a limited number of other benefits, which the C&MD Committee believes are reasonable, appropriate, and consistent with our executive compensation philosophy.
These benefits, which are described in more detail below, are reflected in the “All Other Compensation” column of the Summary Compensation table.
| • | Financial and tax planning. Executives receive a $10,000 cash allowance each December to encourage consultation with knowledgeable financial and tax planning experts who can help them understand the compensation and benefits programs in which they participate. |
| • | Personal use of Company aircraft. Our global security organization regularly evaluates the travel risk for our CEO. As a result of these assessments and based on our security team’s recommendation, our Board of Directors has determined that our CEO must use Company-provided aircraft for all business and personal travel. |
| • | Personal use of Company car and driver. Our CEO is |
| • | Residential security systems. Reimbursement for the installation, maintenance and remote access of residential security systems is provided to select executives, when deemed necessary by our internal global security team. Executives are responsible for paying monthly security monitoring fees, which are not reimbursable. Other than for our CEO, there was no reimbursement for any other NEO in 2023. |
Merck & Co., Inc. 20232024 Proxy Statement
| 60 | ç ç ç ç |
Compensation Discussion and Analysis The Elements of |
2023 Compensation Actions & Scorecard Design
20232024 Compensation Actions
As part of our annual compensation review, the C&MD Committee reviewed and approved target TDC opportunities for oureach executive officers,officer, including our NEOs.NEOs, for 2024. The Board of Directors (excluding Mr. Davis) reviewed and approved Mr. Davis’ target TDC.
Mr. Davis’Davis’s target TDC for 20232024.
Mr. Davis’s target TDC for 2024 increased by 12.3%11.4% based on a review of his performance and the competitive positioning of his compensation relative to the primary pharmaceutical and supplemental peer groups. Consistent with our compensation strategy that supports a pay-for-performance culture, the Board intends to increasecontinue to adjust Mr. Davis’Davis’s target TDC, over timeand when appropriate, increase it to achieve a moreensure it remains competitive position relative to these peer groups assuming continued strongand aligned with the Board’s assessment of his business performance and leadership. As part of Mr. Davis’Davis’s target TDC increase, no change was made to his annual base salary increased by 4.5%, there was no change in hisor target annual cash incentive percent, and hispercent. His target LTI was increased by $1,750,000$2,000,000 to $13,500,000.$15,500,000.
To better align their compensation with the overall market, Ms. Litchfield’s and Dr. Li’sThe target TDC for our other NEOs increased by 38.3%between 1.3% and 31.3%1.5%, respectively. This included market adjustmentsreflecting an increase of 4% to their annual base salaries and increases insalaries. There were no changes to their annual LTI grants. Mr. Guindo was hired on July 1, 2022, and received an annual base salary increase for 2023, increasing his target TDC by 1.5%. Ms. Zachary received an annual base salary increase and an increase to her target annual cash incentive percent from 95% to 100% of base salary, increasing herpercentages or target TDC by 2.8%.LTI grants.
The following table summarizes adjustments made to CEO and other NEO compensation for 2023.2024.
Named Executive Officer | Target Total Direct Compensation | Annual Base Salary
Increase % | Target Annual Incentive
| Target LTI Grant Value
| |||||||||||||||||||||
Davis | + | % | No change | +$ | |||||||||||||||||||||
Litchfield | + | + | No change | No change | |||||||||||||||||||||
| +1.5 | + | No change | ||||||||||||||||||||||
DeLuca | +1.5 | +4.0 | No change | No change | |||||||||||||||||||||
Li | + | + | No change | ||||||||||||||||||||||
| No change | ||||||||||||||||||||||||
|
|
|
2023 Merck Company Scorecard
We believe creating greater accountability for the sustainable delivery of business goals will help drive financial results and long-term shareholder value. As a result, the C&MD Committee approved a new measure in our 2023 Company Scorecard that links the compensation of all of our employees, including our executives, to certain ESG goals. The new design reflects our focus on enabling access to Merck’s innovative portfolio to patients around the world and on the engagement and inclusion of our employees, as illustrated below, each of which is a key strategic ESG priority.
Measure | 2022 Company Scorecard Weighting | 2023 Company Scorecard Weighting | ||
Revenue | 40% | 35% | ||
Pre-Tax Income | 40% | 35% | ||
Pipeline | 20% | 20% | ||
ESG | — | 10% | ||
Merck & Co., Inc. 2023 Proxy Statement
|
|
Other Compensation Practices
Stock Ownership Requirements
The C&MD Committee recognizes the critical role that executive stock ownership has in aligning the interests of management with those of shareholders. As such, we maintain a formal stock ownership policy under whichthat requires the CEO and other senior executives are required to hold a certain amount of Merck common stock in an amount representing a multiple of their base salary for so long as they remain in office. The amount of Merck common stock an executive is required to hold is based on a designated multiple of the executive’s base salary. Until the designated multiple of base salary is reached, executives are required to retain in stock a percentage of the after-tax net proceeds associated with stock option exercises and/or PSU and RSU settlements (100% for the CEO and 75% for the other NEOs). In calculating the attainment of our stock ownership requirements, we exclude (1) unexercised stock options and (2) unvested PSUs and RSUs. All NEOs have met their stock ownership requirements.
Merck & Co., Inc. 2024 Proxy Statement
Compensation Discussion and Analysis The Elements of 2023 Compensation | ÷ ÷ ÷ ÷ | 61 |
The following table sets forth the stock ownership requirements and current stock ownership status as a percentagemultiple of the requirementbase salary for the CEO and other NEOs as of February 28, 2023.29, 2024.
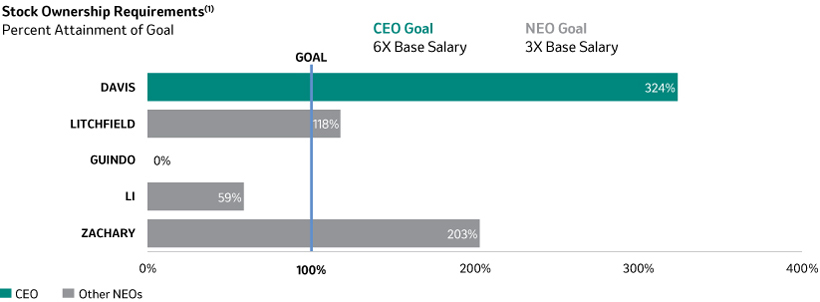

|
Return of Incentive Compensation (“Clawback Policy”)
Under our incentive compensation recoupmentThe C&MD Committee adopted a clawback policy, effective December 1, 2023, in compliance with Rule 10D-1 of the Board will seek reimbursement forSecurities Exchange Act of 1934 and Section 303A.14 of the annual cash incentive and/or LTI awards paidNew York Stock Exchange Listed Company Manual (the “Dodd-Frank Clawback Policy”).
In addition to the executive,Dodd-Frank Clawback Policy, executives are also subject to the recoupment of incentive-based cash compensation, equity-based compensation and any proceeds or earnings received in respect to such compensation, where the BoardC&MD Committee determines that (a) the executive engaged in misconduct or failed to reasonably supervise an employee who engaged in misconduct, that resulted in a (i) material violation of a written Company policy relating to (i) the research, development, manufacturing, sales or marketing of the Company’s products or (ii) conduct detrimental to the Company, including the Company’s overall goodwill or reputation ofto the Company, orand (b) a significant restatement ofnegative impact on the Company’s financial operating results hasor reputation occurred. In the event of a financial restatement, the portion of the annual cash incentive and/or PSUs paid to the executive, in excess of the amount that would have been paid if the financial results were reported accurately, will be recouped.
On October 26, 2022, the SEC adopted final rules implementing the incentive-based compensation recovery provisions of the Dodd-Frank Act. The final rules direct the stock exchanges to establish listing standards requiring listed companies to develop and implement a policy providing for the recovery of erroneously awarded incentive-based compensation received by current or former executive officers and to satisfy related disclosure obligations. We intend to timely amend and restate our clawback policy to reflect these new requirements.
Hedging and Pledging
As part of our insider trading policy, Merck prohibits Directors and management level employees, including officers, from engaging in short sales, publicly traded options, hedging transactions, and pledging of CompanyMerck common stock.
Merck & Co., Inc. 2023 Proxy Statement
|
|
Tax Deductibility of Compensation
In light of the repeal of the performance-based compensation exemption under Section 162(m) of the Internal Revenue Code, the C&MD Committee may authorize compensation that is not deductible if it is determined to be appropriate and in the best interests of the Company and our shareholders.
Merck & Co., Inc. 2024 Proxy Statement
| 62 | ç ç ç ç | Compensation Discussion and Analysis Compensation Risk Assessment |
Compensation Risk Assessment
Our executive compensation program and policies are driven by our business environment and designed to enable us to achieve our mission and adhere to our values. The C&MD Committee and senior management continually evaluate the relationship between risk and reward as it relates to our executive compensation program and have adopted policies and practices that mitigate undue risk while preserving the incentive/variable nature of the compensation. These policies and practices are described in more detail in the Compensation Policies and Practices chart on page 46.48.
In 2022, Merck engaged Pay Governance, a compensation consultant to management, to perform a formal assessment of our executive compensation program, policies, and practices based on generally accepted compensation practices. The results of the assessment were reviewed by FW Cook, the C&MD Committee’s independent compensation consultant, and then discussed with the C&MD Committee in November 2022. The assessment reaffirmed our belief that our compensation programs and policies are structured and operated in a manner that does not create risks that are reasonably likely to have a material adverse effect on our business. In addition to ongoing monitoring of our programs and policies, we are committed to performing formal assessments on a periodic basis. The next formal assessment is scheduled for review and discussion with the C&MD Committee in November 2024.
Compensation and Management Development Committee Report
The C&MD Committee, comprised of independent Directors, reviewed and discussed the above CD&A with management. Based on the review and discussions, the C&MD Committee recommended to our Board of Directors that the CD&A be included in these proxy materials.
Compensation and Management Development Committee
Patricia F. Russo (Chair)
Mary Ellen Coe
Thomas H. Glocer
Risa J. Lavizzo-Mourey, M.D.
Inge G. Thulin
Peter C. Wendell
Merck & Co., Inc. 20232024 Proxy Statement
| ÷ ÷ ÷ ÷ | 63 |
Summary Compensation Table
The following table summarizes the total compensation that was paid or accrued for the Named Executive Officers for the fiscal years ended December 31, 2023, 2022, 2021, and 2020.2021. The Named Executive Officers are the Company’s Chairman and Chief Executive Officer, former Executive Chairman, Chief Financial Officer and the three next most highly compensated executive officers as of December 31, 2022.2023. All amounts in the following table are rounded to the nearest dollar.
Name and Principal Position | Year | Salary ($)(1) | Bonus ($) | Stock Awards ($)(2) | Option Awards ($)(3) | Non-Equity Incentive Compensation ($)(4) | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($)(5) | All Other Compensation ($)(6) | Total ($) | Year | Salary | Bonus | Stock | Option | Non-Equity | Change in | All Other | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Robert M. Davis Chairman, Chief Executive Officer and President |
| 2022 |
| $ | 1,538,613 |
|
| $0 |
|
| $8,868,587 |
| $ | 3,524,995 |
|
| $4,125,150 |
|
| td80,259 | (7) |
| $412,490 |
| $ | 18,650,093 |
|
| 2023 |
| $ | 1,603,091 |
|
| $0 |
|
| $9,997,585 |
| $ | 4,050,000 |
|
| $3,585,300 |
|
| $651,163 | (7) |
| $386,148 |
| $ | 20,273,287 |
| ||||||||||||||||||
| 2021 |
|
| 1,319,959 |
|
| 0 |
|
| 6,324,576 |
|
| 2,760,003 |
|
| 2,834,606 |
|
| 235,640 | (8) |
| 247,337 |
|
| 13,722,121 |
|
| 2022 |
|
| 1,538,613 |
|
| 0 |
|
| 8,868,587 |
|
| 3,524,995 |
|
| 4,125,150 |
|
| 180,259 | (8) |
| 412,490 |
|
| 18,650,093 |
| |||||||||||||||||||
| 2020 |
|
| 1,112,795 |
|
| 0 |
|
| 2,737,406 |
|
| 1,199,772 |
|
| 1,018,194 |
|
| 611,948 |
|
| 159,395 |
|
| 6,839,510 |
|
| 2021 |
|
| 1,319,959 |
|
| 0 |
|
| 6,324,576 |
|
| 2,760,003 |
|
| 2,834,606 |
|
| 235,640 |
|
| 247,337 |
|
| 13,722,121 |
| |||||||||||||||||||
Kenneth C. Frazier Former Executive Chairman |
| 2022 |
|
| 1,151,099 |
|
| 0 |
|
| 5,000,005 |
|
| 0 |
|
| 2,036,028 |
|
| 0 | (9) |
| 323,489 |
|
| 8,510,621 |
| |||||||||||||||||||||||||||||||||||||||||||||
| 2021 |
|
| 1,478,681 |
|
| 0 |
|
| 7,390,092 |
|
| 3,225,004 |
|
| 2,804,094 |
|
| 0 | (10) |
| 299,049 |
|
| 15,196,920 |
| ||||||||||||||||||||||||||||||||||||||||||||||
| 2020 |
|
| 1,702,006 |
|
| 0 |
|
| 10,778,499 |
|
| 4,724,098 |
|
| 2,218,500 |
|
| 2,288,641 |
|
| 376,685 |
|
| 22,088,429 |
| ||||||||||||||||||||||||||||||||||||||||||||||
Caroline Litchfield Executive Vice President and Chief Financial Officer |
| 2022 |
|
| 959,959 |
|
| 0 |
|
| 2,075,595 |
|
| 824,999 |
|
| 1,735,500 |
|
| 0 | (11) |
| 106,385 |
|
| 5,702,438 |
|
| 2023 |
|
| 1,093,063 |
|
| 0 |
|
| 3,147,375 |
|
| 1,275,003 |
|
| 1,665,000 |
|
| 792,534 | (9) |
| 327,410 |
|
| 8,300,385 |
| ||||||||||||||||||
| 2021 |
|
| 805,060 |
|
| 0 |
|
| 1,512,420 |
|
| 660,001 |
|
| 1,184,203 |
|
| 0 | (12) |
| 59,770 |
|
| 4,221,454 |
|
| 2022 |
|
| 959,959 |
|
| 0 |
|
| 2,075,595 |
|
| 824,999 |
|
| 1,735,500 |
|
| 0 | (10) |
| 326,605 |
|
| 5,922,657 |
| |||||||||||||||||||
| 2020 | (17) |
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| 2021 |
|
| 805,060 |
|
| 0 |
|
| 1,512,420 |
|
| 660,001 |
|
| 1,184,203 |
|
| 0 | (11) |
| 434,946 |
|
| 4,596,630 |
| |||||||||||||||||||
Chirfi Guindo Chief Marketing Officer, Human Health |
| 2022 |
|
| 348,077 |
|
| 530,000 | (13) |
| 12,395,556 | (14) |
| 0 |
|
| 502,496 |
|
| 0 | (15) |
| 161,135 |
|
| 13,937,264 |
| |||||||||||||||||||||||||||||||||||||||||||||
| 2021 | (17) |
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
| ||||||||||||||||||||||||||||||||||||||||||||||
| 2020 | (17) |
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
| ||||||||||||||||||||||||||||||||||||||||||||||
Sanat Chattopadhyay Executive Vice President and President, Merck Manufacturing Division |
| 2023 |
|
| 934,923 |
|
| 0 |
|
| 2,443,907 |
|
| 989,997 |
|
| 1,393,873 |
|
| 317,466 |
|
| 160,194 |
|
| 6,240,360 |
| |||||||||||||||||||||||||||||||||||||||||||||
| 2022 | (14) |
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
| ||||||||||||||||||||||||||||||||||||||||||||||
| 2021 | (14) |
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
| ||||||||||||||||||||||||||||||||||||||||||||||
Richard R. DeLuca, Jr. Executive Vice President and President, Merck Animal Health |
| 2023 |
|
| 910,027 |
|
| 0 |
|
| 2,369,846 |
|
| 959,999 |
|
| 1,369,000 |
|
| 470,087 | (12) |
| 118,931 |
|
| 6,197,890 |
| |||||||||||||||||||||||||||||||||||||||||||||
| 2022 | (14) |
| 840,522 |
|
| 0 |
|
| 2,264,301 |
|
| 899,993 |
|
| 1,513,000 |
|
| 0 | (13) |
| 101,011 |
|
| 5,618,828 |
| ||||||||||||||||||||||||||||||||||||||||||||||
| 2021 |
|
| 790,247 |
|
| 0 |
|
| 3,856,115 | (15) |
| 809,999 |
|
| 1,184,000 |
|
| 128,732 |
|
| 71,907 |
|
| 6,841,000 |
| ||||||||||||||||||||||||||||||||||||||||||||||
Dean Li, M.D., Ph.D. Executive Vice President and President, Merck Research Laboratories |
| 2022 |
|
| 1,095,055 |
|
| 0 |
|
| 2,943,574 |
|
| 1,169,998 |
|
| 2,225,000 |
|
| 210,204 |
|
| 122,233 |
|
| 7,766,063 |
|
| 2023 |
|
| 1,368,819 |
|
| 0 |
|
| 4,147,202 |
|
| 1,679,999 |
|
| 2,072,000 |
|
| 339,249 |
|
| 172,146 |
|
| 9,779,414 |
| ||||||||||||||||||
| 2021 |
|
| 937,104 |
|
| 0 |
|
| 2,062,363 |
|
| 900,003 |
|
| 1,400,799 |
|
| 114,593 |
|
| 66,057 |
|
| 5,480,919 |
|
| 2022 |
|
| 1,095,055 |
|
| 0 |
|
| 2,943,574 |
|
| 1,169,998 |
|
| 2,225,000 |
|
| 210,204 |
|
| 122,233 |
|
| 7,766,063 |
| |||||||||||||||||||
| 2020 | (17) |
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| 2021 |
|
| 937,104 |
|
| 0 |
|
| 2,062,363 |
|
| 900,003 |
|
| 1,400,799 |
|
| 114,593 |
|
| 66,057 |
|
| 5,480,919 |
| |||||||||||||||||||
Jennifer Zachary Executive Vice President and General Counsel |
| 2022 |
|
| 970,673 |
|
| 0 |
|
| 2,264,301 |
|
| 899,993 |
|
| 1,648,221 |
|
| 0 | (16) |
| 113,440 |
|
| 5,896,628 |
| |||||||||||||||||||||||||||||||||||||||||||||
| 2021 | (17) |
| 942,325 |
|
| 0 |
|
| 1,856,135 |
|
| 809,999 |
|
| 1,330,516 |
|
| 139,260 | (8) |
| 107,510 |
|
| 5,185,745 |
| ||||||||||||||||||||||||||||||||||||||||||||||
| 2020 |
|
| 913,101 |
|
| 0 |
|
| 1,710,879 |
|
| 749,854 |
|
| 759,347 |
|
| 302,743 |
|
| 121,231 |
|
| 4,557,155 |
| ||||||||||||||||||||||||||||||||||||||||||||||
| (1) | Amounts shown reflect actual base salary earnings and are not reduced to reflect the Named Executive Officers’ elections, if any, to defer receipt of salary into the Merck Deferral Program, an unfunded nonqualified savings plan. |
For more information about deferred amounts, see the Nonqualified Deferred Compensation table and related footnotes on page |
| (2) | The amounts shown in this column represent the full grant date fair value of RSUs and PSUs granted to each of the Named Executive Officers during 2023, 2022, |
The maximum value of the PSU awards granted to the Named Executive Officers during |
Named Executive Officer | Maximum Value
| ||||||
Davis |
| $ | |||||
|
|
| |||||
Litchfield |
|
|
| ||||
|
|
| |||||
DeLuca | 4,739,691 |
| |||||
Li |
|
| |||||
|
|
| |||||
For more information on the awards granted during |
Merck & Co., Inc. 2023 Proxy Statement
|
|
| (3) | The amounts shown in this column represent the full grant date fair value of stock options granted to each of the Named Executive Officers during 2023, 2022 |
Merck & Co., Inc. 2024 Proxy Statement
| 64 | ç ç ç ç | Summary Compensation Table |
For more information on stock options granted during |
| (4) | Represents amounts paid under the EIP. For more information, see the Grants of Plan-Based Awards table and related narrative and footnotes beginning on page |
Amounts shown are not reduced to reflect the Named Executive Officers’ elections, if any, to defer receipt of awards into the Merck Deferral Program. For more information, see the Nonqualified Deferred Compensation table and related notes and narrative on page |
| (5) | Amounts shown are solely an estimate of the aggregate change in actuarial present value of the Named Executive Officers’ accrued benefits under the Company’s pension plans from December 31, |
The Merck Deferral Program, an unfunded nonqualified savings plan, does not provide for above market or preferential earnings. For more information, see the Nonqualified Deferred Compensation table and related notes and narrative on page |
| (6) | See the All Other Compensation table on page |
| (7) |
|
| (8) | For 2022, the change in value compared to the previous year is less primarily due to an increase in discount rates as of December 31, 2022. |
|
|
| (10) |
|
For 2022, the U.S. change in pension value is positive; however, the U.K. change in pension value is negative primarily due to an increase in discount rates, resulting in an aggregate negative change in pension value that is reported as $0 in accordance with SEC rules. |
For 2021, the U.S. change in pension value is positive; however, the U.K. change in pension value is negative due to an increase in discount rates and change in mortality assumptions, resulting in an aggregate negative change in pension value that is reported as $0 in accordance with SEC rules. |
|
|
|
For 2022, the change in value for |
|
| (15) | Includes value of $2,000,000 RSU retention grant that will vest on May 4, 2024, subject to his continued employment. |
Merck & Co., Inc. 20232024 Proxy Statement
Summary Compensation Table | ÷ ÷ ÷ ÷ | 65 |
All Other Compensation
Name | Year | Financial/Tax Counseling & Tax Preparation Services ($)(1) | Company Aircraft ($)(2) | Company Car and Driver ($)(3) | Installation, Maintenance and Remote Access ($)(4) | Relocation Expense ($) | Savings Plan Company Match and Credits ($)(6) | Total ($) | Year | Financial/Tax Counseling & Tax Preparation Services ($)(1) | Company Aircraft ($)(2) | Company Car and Driver ($)(3) | Installation, Maintenance and Remote Access of Home Security ($)(4) | Relocation Expense and Tax Equalization ($) | Savings Plan Company Match and Credits ($)(5) | Total ($) | ||||||||||||||||||||||||||||||||||||||||||||||||
Davis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Davis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Davis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Davis | 2022 | $10,000 | $191,073 | $8,113 | $6,689 | $0 | $196,615 | $412,490 | 2023 | $10,000 | $91,519 | $15,224 | $11,824 | $0 | $257,580 | $386,148 | ||||||||||||||||||||||||||||||||||||||||||||||||
| 2021 | 10,000 | 94,552 | 11,353 | 26,258 | 0 | 105,174 | 247,337 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 2020 | 10,000 | 0 | 3,620 | 0 | 0 | 145,775 | 159,395 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2022 | 10,000 | 191,073 | 8,113 | 6,689 | 0 | 196,615 | 412,490 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Frazier | 2022 | 10,000 | 115,697 | 15,163 | 4,954 | 0 | 177,675 | 323,489 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2021 | 10,000 | 93,769 | 25,262 | 3,966 | 0 | 166,052 | 299,049 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 2020 | 10,000 | 21,983 | 50,572 | 7,637 | 0 | 286,493 | 376,685 | 2021 | 10,000 | 94,552 | 11,353 | 26,258 | 0 | 105,174 | 247,337 | ||||||||||||||||||||||||||||||||||||||||||||||||
Litchfield | 2022 | 10,000 | 0 | 0 | 0 | 0 | 96,385 | 106,385 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Litchfield | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Litchfield | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Litchfield | 2023 | 10,000 | 0 | 0 | 0 | 190,245 | (7) | 127,165 | 327,410 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2021 | 10,000 | 0 | 0 | 0 | 0 | 49,770 | 59,770 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 2020 | (7) | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2022 | 10,000 | 0 | 0 | 0 | 220,220 | (7) | 96,385 | 326,605 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Guindo | 2022 | 10,000 | 0 | 0 | 0 | 138,085 | (5) | 13,050 | 161,135 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 2021 | 10,000 | 0 | 0 | 0 | 375,176 | (7) | 49,770 | 434,946 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | 2023 | 10,000 | 0 | 36,044 | 0 | 0 | 114,150 | 160,194 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2022 | (6) | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 2021 | (6) | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DeLuca | 2023 | 10,000 | 0 | 0 | 0 | 0 | 108,931 | 118,931 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2022 | (6) | 10,000 | 0 | 0 | 0 | 0 | 91,011 | 101,011 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2021 | (7) | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 2020 | (7) | — | — | — | — | — | — | — | 2021 | 10,000 | 0 | 0 | 0 | 0 | 61,907 | 71,907 | |||||||||||||||||||||||||||||||||||||||||||||||
Li | 2022 | 10,000 | 0 | 0 | 0 | 0 | 112,233 | 122,233 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Li | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Li | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Li | 2023 | 10,000 | 0 | 579 | 0 | 0 | 161,567 | 172,146 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2021 | 10,000 | 0 | 0 | 0 | 0 | 56,057 | 66,057 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 2020 | (7) | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2022 | 10,000 | 0 | 0 | 0 | 0 | 112,233 | 122,233 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Zachary | 2022 | 10,000 | 0 | 0 | 0 | 0 | 103,440 | 113,440 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2021 | (7) | 10,000 | 18,537 | 351 | 0 | 0 | 78,623 | 107,510 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 2020 | 10,000 | 0 | 0 | 0 | 0 | 111,231 | 121,231 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 2021 | 10,000 | 0 | 0 | 0 | 0 | 56,057 | 66,057 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (1) | The Named Executive Officers receive a cash allowance each December for financial and tax planning benefits. |
| (2) | The value of any personal use of Company aircraft by the Named Executive Officers is based on the aggregate incremental per-hour cost based on the flight time flown from origination to destination and a return to point of origination without passengers, when applicable. This benefit is taxable to the Named Executive Officers. As further described in the Other Employee Benefits section on page |
| (3) | The value of any personal use of Company car and driver by the Named Executive Officers is based on the recipient’s cost if equivalent assets were used independent of the Company. This benefit is taxable to the Named Executive Officers. |
The incremental cost calculation for personal use of Company car and driver by the Named Executive Officers includes driver overtime, meals, travel pay, maintenance and fuel costs. Personal use of a car and driver is also provided to a select number of other executives, primarily for commuting purposes, as further described in the Other Employee Benefits section on page 59.
|
| (4) | Installation, maintenance, and remote access of home security are valued at actual costs billed by outside vendors. |
| (5) |
|
The Named Executive Officers received Company matching contributions equal to 75% of the first 6% of eligible compensation contributed (up to the IRS limit for qualified savings plans) to the Merck U.S. Savings Plan and a 4.5% credit of eligible compensation in excess of the IRS limit to the Named Executive Officers’ accounts under the Merck Deferral Program. |
|
| (7) | Prior to her appointment as our Chief Financial Officer, Ms. Litchfield served on an expatriate assignment that entitled her to receive certain tax equalization benefits, consistent with the Company’s standard practices for employees serving in an expatriate capacity. The tax equalization benefits are designed to mitigate the impact of an expatriate assignment by covering tax expense in excess of what the employee would have incurred had the employee remained in their home country. |
Merck & Co., Inc. 20232024 Proxy Statement
| 66 | ç ç ç ç |
|
CEO Pay Ratio
Introduction
The following is a disclosure of (1) total annual compensation for our CEO, (2) the median total annual compensation for our employees globally, excluding our CEO and (3) the ratio of those two numbers.
Median Total Annual Compensation
We used base salary as of October 31, 20222023 to identify the employee with the median total annual compensation (excluding our CEO). For this purpose, we annualized base salary for all full and part-time employees (excluding our CEO) hired after January 1, 20222023 and employed as of October 31, 2022.2023. We converted foreign currency to U.S. dollars using a twelve-month average exchange rate between November 1, 20212022 through October 31, 2022.2023.
Exemptions
Total Employees Before and After De Minimis ExemptionEmployee Population
Merck’s employee population as of October 31, 20222023 included 27,850 (38%29,358 (39%) employees in the United States and 45,282 (62%46,859 (61%) employees outside the United States. After excluding 3,6563,810 employees in 2515 countries, as detailed in the table below and up to the 5% limit allowable under the SEC disclosure rules, we identified our median employee from a group of approximately 69,47672,407 employees globally.
Excluded Under De Minimis Exemption
Country | Number of Employees | Country | Number of Employees | Number of Employees | Country | Number of Employees | ||||||||||||||||||||||
Algeria | 33 | Latvia | 13 | |||||||||||||||||||||||||
Bahrain | 2 | Luxembourg | 2 | |||||||||||||||||||||||||
Belarus | 2 | Malaysia | 413 | |||||||||||||||||||||||||
Algeria | ||||||||||||||||||||||||||||
Algeria | ||||||||||||||||||||||||||||
Algeria | 35 | Jordan | 23 | |||||||||||||||||||||||||
Bosnia and Herzegovina | 8 | Nigeria | 1 | |||||||||||||||||||||||||
Bosnia and Herzegovina | ||||||||||||||||||||||||||||
Bosnia and Herzegovina | ||||||||||||||||||||||||||||
Bosnia and Herzegovina | 8 | Latvia | 18 | |||||||||||||||||||||||||
Bulgaria | 60 | North Macedonia | 4 | |||||||||||||||||||||||||
Colombia | ||||||||||||||||||||||||||||
Colombia | ||||||||||||||||||||||||||||
Colombia | ||||||||||||||||||||||||||||
Colombia | 1,040 | Malaysia | 371 | |||||||||||||||||||||||||
Dominican Republic | 9 | Oman | 3 | |||||||||||||||||||||||||
Ecuador | 52 | Philippines | 231 | |||||||||||||||||||||||||
Dominican Republic | ||||||||||||||||||||||||||||
Dominican Republic | ||||||||||||||||||||||||||||
Dominican Republic | 7 | Nigeria | 1 | |||||||||||||||||||||||||
Egypt | 185 | Serbia | 56 | |||||||||||||||||||||||||
Egypt | ||||||||||||||||||||||||||||
Egypt | ||||||||||||||||||||||||||||
Egypt | 185 | North Macedonia | 3 | |||||||||||||||||||||||||
Estonia | 11 | Thailand | 274 | |||||||||||||||||||||||||
Honduras | ||||||||||||||||||||||||||||
Honduras | ||||||||||||||||||||||||||||
Honduras | ||||||||||||||||||||||||||||
Honduras | 5 | Turkey | 533 | 5 | Philippines | 206 | ||||||||||||||||||||||
India | 1,235 | Uruguay | 73 | |||||||||||||||||||||||||
India | ||||||||||||||||||||||||||||
India | ||||||||||||||||||||||||||||
India | 1,489 | Vietnam | 253 | |||||||||||||||||||||||||
Indonesia | 174 | Vietnam | 247 | |||||||||||||||||||||||||
Jordan | 30 | |||||||||||||||||||||||||||
Indonesia | ||||||||||||||||||||||||||||
Indonesia | ||||||||||||||||||||||||||||
Indonesia | 166 | |||||||||||||||||||||||||||
TOTAL | 3,656 | |||||||||||||||||||||||||||
TOTAL | ||||||||||||||||||||||||||||
TOTAL | ||||||||||||||||||||||||||||
TOTAL | 3,810 | |||||||||||||||||||||||||||
The Ratio
The total annual compensation of our median employee as calculated under the Summary Compensation table requirements for calculating total annual compensation was $98,943$110,827 comprised of base salary, annual cash incentive, savings plan company match, and change in pension value. The total annual compensation for our CEO was $18,650,093.$20,273,287. A reasonable estimation of the ratio of our CEO’s compensation to our median employee’s compensation is 188183 to 1.
Under the SEC rules, companies may identify the median total annual compensation using a wide variety of methods, including reasonable assumptions and estimations. It is, therefore, difficult to compare Merck’s ratio to the ratios of other companies.
Merck & Co., Inc. 20232024 Proxy Statement
÷ ÷ ÷ ÷ | 67 |
Company Scorecard Metrics | PSU Program Metrics | ||||
| Revenue | Relative Total Shareholder Return | ||||
Pre-Tax Income | Non-GAAP Earnings Per Share (EPS) | ||||
| Pipeline | |||||
| Sustainability | |||||

| 68 | ||||||||||
ç ç ç ç | Pay Versus Performance Pay Versus Performance Table |
Summary Compensation Table Total for CEO (Davis) | Summary Compensation Table Total for CEO (Frazier) | Compensation Actually Paid to CEO (Davis) (4) | Compensation Actually Paid to CEO (Frazier) (4) | Average Summary Compensation Table Total for Non-CEO NEOs | Average Compensation Actually Paid to Non-CEO NEOs (4) | Value of Initial Fixed $100 Investment Based on | ||||||||||||||||||||||||||||||||||
Fiscal Year | Total Shareholder Return (5) | Peer Group Total Shareholder Return (6) | GAAP Net Income ($000’s) | Non-GAAP EPS (7) | ||||||||||||||||||||||||||||||||||||
2022 (1) | $18,650,093 | — | $52,474,235 | $8,362,603 | $22,297,770 | $141.10 | $138.10 | $14,519,000 | $7.48 | |||||||||||||||||||||||||||||||
2021 (2) | 13,722,121 | $15,196,920 | 14,390,893 | $13,328,924 | 6,616,111 | 6,911,156 | 94.50 | 130.80 | 12,345,000 | $5.37 | ||||||||||||||||||||||||||||||
2020 (3) | — | 22,088,429 | 10,578,487 | 6,041,722 | 3,582,756 | 92.80 | 107.10 | 4,519,000 | $2.97 | |||||||||||||||||||||||||||||||
Summary Compensation Table Total for CEO (Davis) | Summary Compensation Table Total for CEO (Frazier) | Compensation Actually Paid to CEO (Davis) (5) | Compensation Actually Paid to CEO (Frazier) (5) | Average Summary Compensation Table Total for Non-CEO NEOs | Average Compensation Actually Paid to Non-CEO NEOs (5) | Value of Initial Fixed $100 Investment Based on | ||||||||||||||||||||||||||||||||||
Fiscal Year | Total Shareholder Return (6) | Peer Group Total Shareholder Return (7) | GAAP Net Income ($ 000’s) | Non-GAAP EPS (8) | ||||||||||||||||||||||||||||||||||||
2023 (1) | $20,273,287 | — | $26,701,326 | — | $7,629,512 | $9,360,664 | $142.53 | $142.00 | $364,655 | $1.51 | ||||||||||||||||||||||||||||||
2022 (2) | 18,650,093 | — | 52,474,235 | — | 8,406,647 | 22,341,814 | 141.12 | 138.11 | 14,519,000 | 7.48 | ||||||||||||||||||||||||||||||
2021 (3) | 13,722,121 | 15,196,920 | 14,390,893 | 13,328,924 | 6,709,905 | 7,004,950 | 94.47 | 130.44 | 12,345,000 | 5.37 | ||||||||||||||||||||||||||||||
2020 (4) | — | 22,088,429 | — | 10,578,487 | 6,041,722 | 3,582,756 | 92.79 | 106.53 | 4,519,000 | 2.97 | ||||||||||||||||||||||||||||||
| (1) | Non-CEO NEOs for 2023 are Ms. Litchfield, Mr. Chattopadhyay, Mr. DeLuca, and Dr. Li. |
| (2) | Non-CEO NEOs for 2022 are Mr. Frazier, Ms. Litchfield, Mr. Guindo, Dr. Li, |
Non-CEO NEOs for 2021 are Ms. Litchfield, Mr. Clyburn, Mr. DeLuca, and Dr. Li. As a result of Mr. Frazier’s retirement as CEO, effective June 30, 2021, and Mr. |
Non-CEO NEOs for 2020 are Mr. Davis, Mr. Chattopadhyay, Dr. Perlmutter, and Ms. Zachary. Since Mr. Frazier was CEO for all of 2020, only his compensation is shown in the CEO SCT and CAP columns. |
| See following table for additional details on the calculation of the CAP value. |
S-K. |
Peer Group TSR assumes an initial $100 investment in Merck’s primary peer group (market cap-weighted). As described on page |
The SEC requires the disclosure of a company selected measure, representing the most important financial metric used for determining CAP for the current fiscal year. As described in more detail on page non-GAAP EPS. Refer to Appendix A on page non-GAAP financial measures. Appendix B on page non-GAAP results for incentive plans purposes. |
Pay Versus Performance Pay Versus Performance Table | ÷ ÷ ÷ ÷ | 69 |
Summary Compensation Table Total Compensation | Value of Pension Benefits Deducted from SCT (1) | Value of Equity Deducted from SCT | Value of Pension Benefits Per CAP Definition (2) | Fair Value of Equity Compensation Granted in Current Year (3) | Year-Over-Year Change in Fair Value of Unvested Equity (4) | Year-Over- Year Change in Fair Value of Equity that Vested During the Year (5) | Value of Dividends Accrued or Paid on Stock Awards (6) | Compensation Actually Paid | Summary Compensation Table Total Compensation | Value of Pension Benefits Deducted from SCT (1) | Value of Equity Deducted from SCT | Value of Pension Benefits Per CAP Definition (2) | Fair Value of Equity Compensation Granted in Current Year (3) | Year-Over-Year Change in Fair Value of Unvested Equity (4) | Change in Fair Value of Equity that Vested During the Year (5) | Value of Dividends Accrued or Paid on Stock Awards (6) | Compensation Actually Paid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
CEO (Davis) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2023 | $20,273,287 | $651,163 | $14,047,586 | $271,130 | $18,028,095 | $(97,780) | $1,522,952 | $1,402,391 | $26,701,326 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2022 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2022 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2022 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2022 | $18,650,093 | $180,259 | $12,393,581 | $280,085 | $26,824,642 | $14,171,478 | $3,931,065 | $1,190,712 | $52,474,235 | 18,650,093 | 180,259 | 12,393,581 | 280,085 | 26,824,642 | 14,171,478 | 3,931,065 | 1,190,712 | 52,474,235 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 | 13,722,121 | 235,640 | 9,084,579 | 265,823 | 10,649,030 | (669,339 | ) | (661,118 | ) | 404,596 | 14,390,893 | 13,722,121 | 235,640 | 9,084,579 | 265,823 | 10,649,030 | (669,339 | ) | (661,118 | ) | 404,596 | 14,390,893 | ||||||||||||||||||||||||||||||||||||||||||||||||||
CEO (Frazier) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 | 15,196,920 | 0 | 10,615,096 | 418,183 | 12,443,073 | (2,633,787 | ) | (2,435,189 | ) | 954,819 | 13,328,924 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 | 15,196,920 | — | 10,615,096 | 418,183 | 12,443,073 | (2,633,787 | ) | (2,435,189 | ) | 954,819 | 13,328,924 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2020 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2020 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2020 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2020 | 22,088,429 | 2,288,641 | 15,502,597 | 379,037 | 17,150,711 | (5,340,113 | ) | (7,290,425 | ) | 1,382,086 | 10,578,487 | 22,088,429 | 2,288,641 | 15,502,597 | 379,037 | 17,150,711 | (5,340,113 | ) | (7,290,425 | ) | 1,382,086 | 10,578,487 | ||||||||||||||||||||||||||||||||||||||||||||||||||
Average Non-CEO NEOs | Average Non-CEO NEOs | Average Non-CEO NEOs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2023 | 7,629,512 | 479,834 | 4,253,332 | 152,186 | 5,458,528 | (43,471 | ) | 475,952 | 421,122 | 9,360,664 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2022 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2022 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2022 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2022 | 8,362,603 | 42,041 | 5,514,804 | 122,447 | 9,133,936 | 6,336,440 | 3,346,846 | 552,343 | 22,297,770 | 8,406,647 | 42,041 | 5,514,804 | 122,447 | 9,133,936 | 6,336,440 | 3,346,846 | 552,343 | 22,341,814 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 | 6,616,111 | 107,732 | 4,261,734 | 122,147 | 4,829,729 | (247,588 | ) | (208,749 | ) | 168,972 | 6,911,156 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2021 | 6,709,905 | 107,732 | 4,261,734 | 122,147 | 4,829,729 | (247,588 | ) | (208,749 | ) | 168,972 | 7,004,950 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2020 | 6,041,722 | 498,315 | 3,518,845 | 157,683 | 3,892,938 | (1,222,908 | ) | (1,583,911 | ) | 314,391 | 3,582,756 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2020 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2020 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2020 | 6,041,722 | 498,315 | 3,518,845 | 157,683 | 3,892,938 | (1,222,908 | ) | (1,583,911 | ) | 314,391 | 3,582,756 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (1) | Represents the aggregate change in actuarial present value of the NEOs’ accrued benefits under the Company’s pension plans. The change in pension value for Mr. Frazier is negative in 2021, primarily due to age and an increase in discount rates. In accordance with SEC rules, a $0 value is reported rather than a negative amount. |
| (2) | These amounts represent the present value of expected pension benefit |
| (3) | These amounts represent the fair value as of the indicated fiscal year-end of the outstanding and unvested stock and option awards granted during such fiscal year, calculated in accordance with the methodology used for financial reporting purposes. The fair value differs from the value in the SCT because for purposes of CAP the fair value for equity granted in the current year is determined as of the last day of the applicable year. Fair values in the SCT are determined as of the grant date. |
| (4) | These amounts represent the change in fair value during the indicated fiscal year of each stock and option award that was granted in a prior fiscal year and that remained outstanding and unvested as of the last day of the indicated fiscal year, calculated in accordance with the methodology used for financial reporting purposes and, for awards subject to performance-based vesting conditions, based on an estimate of the probable outcome of such performance-based vesting conditions as of the last day of the fiscal year. |
| (5) | These amounts represent the change in fair value, measured from the prior fiscal year-end to the vesting date, of each stock and option award that was granted in a prior fiscal year and which vested during the indicated fiscal year, calculated in accordance with the methodology used for financial reporting purposes. |
| (6) | These amounts represent the dollar value of any dividends or other earnings accrued or paid on stock or option awards in the applicable fiscal year or for awards that vested in the fiscal year, prior to the vesting date, that are not otherwise reflected in the fair value of such awards or included in any other component of total compensation for the applicable fiscal year. |
| 70 | ||||||||||
ç ç ç ç | Pay Versus Performance Pay Versus Performance Table |

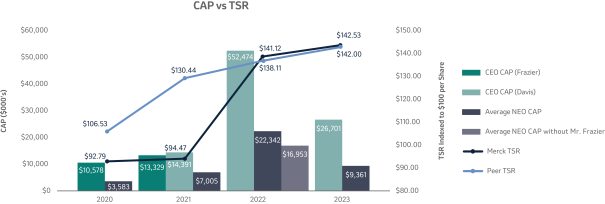
Reporting Year | Beginning | End | Number of Years | |||||||||||||||||||||||
| 12/31/2019 | 12/31/2023 | 4 years | ||||||||||||||||||||||||
| 2022 | 12/31/2019 | 12/31/2022 | 3 years | |||||||||||||||||||||||
| 2021 | 12/31/2019 | 12/31/2021 | 2 years | |||||||||||||||||||||||
| 2020 | 12/31/2019 | 12/31/2020 | 1 year | |||||||||||||||||||||||
Pay Versus Performance Pay Versus Performance Table | ÷ ÷ ÷ ÷ | 71 |
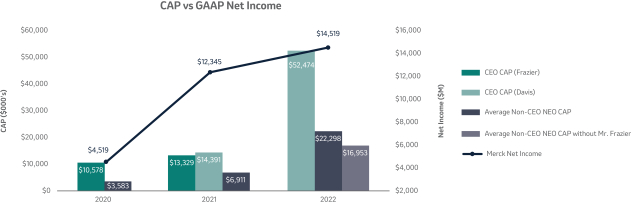
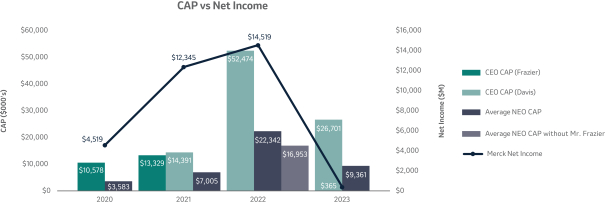
| 72 | ç ç ç ç | Pay Versus Performance Pay Versus Performance Table |
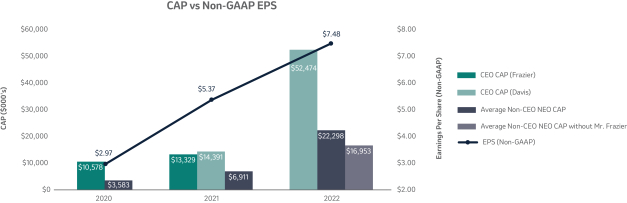
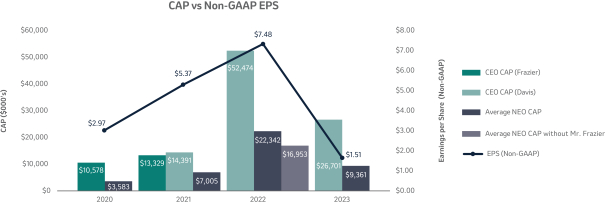
|
÷ ÷ ÷ | 73 |
Grants of Plan-Based Awards
The following table provides information concerning each award made in 20222023 to the Named Executive Officers under any incentive plan.
Grants of Plan-Based Awards for Fiscal Year Ended December 31, 20222023
| Estimated Future Payouts Under Non-Equity Incentive Plan Awards | Estimated Future Payouts Under Equity Incentive Plan Awards | All Other Stock Awards: Number of of Stock or Units (#)(3) | All Other Option Awards: Number of Securities Underlying Options (#)(4) | Exercise or Base Price of Option Awards ($/Sh)(4) | Grant Date Fair Value of Stock and Option Awards ($)(5) |
Estimated Future Payouts |
Estimated Future Payouts | All Other | All Other | Exercise | Grant Date | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Name | Grant Date | Approval Date | Award Type | Thres- hold ($)(1) | Target ($)(1) | Maximum ($)(1) | Thres- hold (#)(2) | Target (#)(2) | Maximum (#)(2) | Grant Date | Approval | Award | Thres- | Target | Maximum | Thres- | Target | Maximum | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Davis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Davis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Davis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Davis | 03/31/22 | 03/21/22 | PSU |
|
|
| 0 | 100,244 | 200,488 |
|
|
| $8,868,587 | 3/31/23 | 3/27/23 | PSU |
|
|
| 0 | 88,824 | 177,648 |
|
|
| $9,997,585 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 05/03/22 | 01/24/22 | Options |
|
|
|
|
|
|
| 228,155 | $87.10 | 3,524,995 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
| EIP | $0 | $ | 2,317,500 | $ | 4,635,000 |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5/2/23 | 1/23/23 | Options |
|
|
|
|
|
|
| 186,722 | $117.89 | 4,050,000 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Frazier | 05/03/22 | 03/21/22 | RSU |
|
|
|
|
|
| 28,703 |
|
| 2,500,031 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 08/03/22 | 07/25/22 | RSU |
|
|
|
|
|
| 28,532 | (6) |
|
| 2,499,974 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
| EIP | 0 | 1,143,836 | 2,287,672 |
|
|
|
|
|
|
|
|
| EIP | $0 | $ | 2,422,500 | $ | 4,845,000 |
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Litchfield | 03/31/22 | 03/21/22 | PSU |
|
|
| 0 | 23,461 | 46,922 |
|
|
| 2,075,595 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Litchfield | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Litchfield | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Litchfield | 3/31/23 | 3/27/23 | PSU |
|
|
| 0 | 27,963 | 55,926 |
|
|
| 3,147,375 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 05/03/22 | 01/24/22 | Options |
|
|
|
|
|
|
| 53,398 | 87.10 | 824,999 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
| EIP | 0 | 975,000 | 1,950,000 |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5/2/23 | 1/23/23 | Options |
|
|
|
|
|
|
| 58,783 | 117.89 | 1,275,003 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Guindo | 08/03/22 | 06/14/22 | PSU |
|
|
| 0 | 28,532 | 57,064 |
|
|
| 2,695,584 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
| EIP | 0 | 1,125,000 | 2,250,000 |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | 3/31/23 | 3/27/23 | PSU |
|
|
| 0 | 21,713 | 43,426 |
|
|
| 2,443,907 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5/2/23 | 1/23/23 | Options |
|
|
|
|
|
|
| 45,643 | 117.89 | 989,997 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
| EIP | 0 | 941,806 | 1,883,612 |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DeLuca | 3/31/23 | 3/27/23 | PSU |
|
|
| 0 | 21,055 | 42,110 |
|
|
| 2,369,846 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5/2/23 | 1/23/23 | Options |
|
|
|
|
|
|
| 44,260 | 117.89 | 959,999 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 08/03/22 | 06/14/22 | RSU |
|
|
|
|
|
| 110,705 |
|
| 9,699,972 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
| EIP | 0 | 282,301 | 564,602 |
|
|
|
|
|
|
|
|
| EIP | 0 | 925,000 | 1,850,000 |
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Li | 03/31/22 | 03/21/22 | PSU |
|
|
| 0 | 33,272 | 66,544 |
|
|
| 2,943,574 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Li | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Li | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Li | 3/31/23 | 3/27/23 | PSU |
|
|
| 0 | 36,846 | 73,692 |
|
|
| 4,147,202 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 05/03/22 | 01/24/22 | Options |
|
|
|
|
|
|
| 75,728 | 87.10 | 1,169,998 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
| EIP | 0 | 1,250,000 | 2,500,000 |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5/2/23 | 1/23/23 | Options |
|
|
|
|
|
|
| 77,455 | 117.89 | 1,679,999 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Zachary | 03/31/22 | 03/21/22 | PSU |
|
|
| 0 | 25,594 | 51,188 |
|
|
| 2,264,301 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 05/03/22 | 01/24/22 | Options |
|
|
|
|
|
|
| 58,252 | 87.10 | 899,993 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
| EIP | 0 | 925,967 | 1,851,934 |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
| EIP | 0 | 1,400,000 | 2,800,000 |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (1) | Amounts represent awards under the EIP, which equal a specified percentage of base salary as in effect on December 31, |
| (2) | The payout of PSUs can range from zero for below threshold performance to a maximum of 200% of target, depending on the level of achievement of the applicable performance goals. For more information on PSUs, see the PSU Program section on page |
| (3) | For more information on RSUs, see the narrative to the Grants of Plan-Based Awards table on |
| (4) | Stock options generally vest and become exercisable in equal installments on the first, second and third anniversaries of the grant date. For more information on stock options granted to the NEOs in |
| (5) | This column represents the full grant date fair value of PSUs, RSUs and stock options granted to each of the NEOs, as calculated in accordance with FASB ASC Topic 718. These amounts do not represent the actual value realized by the NEOs during |
|
Merck & Co., Inc. 20232024 Proxy Statement
| 74 |
ç ç ç |
Grants of Plan-Based Awards Narrative Information Relating to the Grants of Plan-Based Awards Table |
Narrative Information Relating to the Grants of Plan-Based Awards Table
General Information Regarding the EIP
The EIP is a shareholder-approved plan that is administered by the C&MD Committee. It is designed to provide cash awards to employees who are subject to Section 16 of the Securities Exchange Act of 1934, as amended, as follows:
Each executive officer is assigned a target award opportunity that is expressed as a multiple of salary.
The Company performance component (as reflected by the Company Scorecard) is multiplied by the target award opportunity.
The Company performance component can range between 50% and 200% of target.
If the combined results of the three metrics do not total at least 50, no payout will be made.
General Information Regarding Long-Term Incentives
Stock Options
Stock options enable executives to share in the financial gain derived from the potential appreciation in stock price from the date the option is granted until the date the option is exercised. The exercise price of a stock option is set as the closing price of Merck common stock as reported on the NYSE on the grant date.
Subject to their terms, stock options generally vest and become exercisable in equal installments on the first, second and third anniversaries of the grant date and expire on the day before the tenth anniversary of the grant date.
RSUs
RSUs, subject to their terms, generally vest and become payable in equal installments in shares of Merck common stock on the first, second and third anniversaries of the grant date. Dividend equivalents are accrued and paid out in cash if, and when, the RSUs vest.
For Mr. Frazier in 2022, while there had not been any definitive timing for Mr. Frazier’s retirement as Executive Chairman at the time his 2022 LTI grant was made, the Board determined that his continued service as Executive Chairman would be for a transition period and, as such, provided Mr. Frazier’s 2022 LTI grant in the form of two RSU awards. Under the terms of the May 3, 2022 grant, the RSU award vests in full on May 3, 2023 provided that Mr. Frazier was employed through June 30, 2022, which he was. Under the terms of the August 3, 2022 grant, the RSU award vests in full on August 3, 2023 provided that Mr. Frazier was employed through December 31, 2022, and if not, the award is to be prorated based on the number of complete months worked from July 1, 2022 divided by 6. Mr. Frazier retired on November 30, 2022 and, as a result, his award was pro-rated accordingly.
PSUs
PSUs, subject to their terms, generally vest and become payable in shares of Merck common stock at the end of a three-year performance period provided that minimum performance goals are met. Failure to attain the minimum performance goal results in forfeiture of the shares applicable to the respective award opportunity. PSU awards for continuing executives and performance goals are approved by the C&MD Committee within the first 90 days of the applicable performance cycle.
For PSUs granted in 2022,2023, final awards will be determined based on the following:
50% of the award will be determined by the Company’s cumulative EPS versus target for the three-year performance period (2022-2024)(2023-2025).
50% of the award will be determined by the Company’s average annual total shareholder returnTSR (inclusive of reinvested dividends) relative to the median total shareholder returnTSR for our primary peer group for the three-year performance period (2022-2024)(2023-2025).
Payouts can range from zero (for below threshold performance) to a maximum of 200% of target.
Dividend equivalents are accrued and paid in shares if, and when, the PSUs vest, and are only applied to the portion of the award that is earned.
Merck & Co., Inc. 20232024 Proxy Statement
|
÷ ÷ ÷ | 75 |
Outstanding Equity Awards
The following table provides details about each outstanding equity award held by the Named Executive Officers as of December 31, 2022.2023.
Outstanding Equity Awards for Fiscal Year Ended December 31, 20222023
| Option Awards | Stock Awards | Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Name | Number of Securities Underlying Unexercised Options Exercisable (#)(1) | Number of Securities Underlying Unexercised Options Unexercisable (#)(1) | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Grant Date | Option Exercise Price ($) | Vesting Date | Option Expiration Date | Number of Shares or Units (#)(2) | Market Value of Shares or Units of Stock That Have Not Vested ($)(2)(5) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(5) | Number of | Number of | Equity | Grant | Option | Vesting | Option | Number | Market | Equity | Equity | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Davis | 143,329 |
|
| 05/04/18 | 56.04 | 05/04/19 | 05/03/28 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 116,370 |
|
| 05/03/19 | 77.62 | 05/03/20 | 05/02/29 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 83,018 | 41,511 |
| 05/01/20 | 75.36 | 05/01/21 | 04/30/30 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 96,756 | 193,516 |
| 05/04/21 | 73.73 | 05/04/22 | 05/03/31 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 228,155 |
| 05/03/22 | 87.10 | 05/03/23 | 05/02/32 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| 172,204 | (3) | $19,106,033 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| 200,488 | (4) | $22,244,143 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Frazier | 551,360 |
|
| 05/05/17 | 62.07 | 05/05/18 | 05/04/27 | (8) |
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Davis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Davis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Davis | 116,370 |
|
| 05/03/19 | $77.62 | 05/03/20 | 05/02/29 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 490,337 |
|
| 05/04/18 | 56.04 | 05/04/19 | 11/30/27 | (8) |
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 436,390 |
|
| 05/03/19 | 77.62 | 05/03/20 | 11/30/27 | (8) |
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 124,529 |
|
| 05/01/20 | 75.36 | 05/01/21 | 04/30/30 |
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 326,888 | 163,446 | (6) |
| 05/01/20 | 75.36 | 05/01/21 | 11/30/27 | (8) |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 113,059 | 226,118 | (7) |
| 05/04/21 | 73.73 | 05/04/22 | 11/30/27 | (8) |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
| 28,703 | $3,184,597 |
|
| 193,512 | 96,760 |
| 05/04/21 | 73.73 | 05/04/22 | 05/03/31 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
| 23,776 | $2,637,947 |
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| 201,214 | (3)(9) | $22,324,693 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 76,051 | 152,104 |
| 05/03/22 | 87.10 | 05/03/23 | 05/02/32 |
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 186,722 |
| 05/02/23 | 117.89 | 05/02/24 | 05/01/33 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| 200,488 | (3) | $21,857,202 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| 177,648 | (4) | 19,367,185 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Litchfield | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Litchfield | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Litchfield | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Litchfield | 38,291 |
|
| 05/01/15 | 58.08 | 05/01/16 | 04/30/25 |
|
|
|
|
| 38,291 |
|
| 05/01/15 | 58.08 | 05/01/16 | 04/30/25 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 41,997 |
|
| 05/10/16 | 53.06 | 05/10/17 | 05/09/26 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 26,465 |
|
| 05/05/17 | 62.07 | 05/05/18 | 05/04/27 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 41,997 |
|
| 05/10/16 | 53.06 | 05/10/17 | 05/09/26 |
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 22,630 |
|
| 05/04/18 | 56.04 | 05/04/19 | 05/03/28 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17,455 |
|
| 05/03/19 | 77.62 | 05/03/20 | 05/02/29 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13,540 | 6,773 |
| 05/01/20 | 75.36 | 05/01/21 | 04/30/30 |
|
|
|
|
| 26,465 |
|
| 05/05/17 | 62.07 | 05/05/18 | 05/04/27 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 23,137 | 46,276 |
| 05/04/21 | 73.73 | 05/04/22 | 05/03/31 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 53,398 |
| 05/03/22 | 87.10 | 05/03/23 | 05/02/32 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 22,630 |
|
| 05/04/18 | 56.04 | 05/04/19 | 05/03/28 |
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
| 866 | $96,082 |
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| 41,180 | (3) | $4,568,921 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| 46,922 | (4) | $5,205,995 | 17,455 |
|
| 05/03/19 | 77.62 | 05/03/20 | 05/02/29 |
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 20,313 |
|
| 05/01/20 | 75.36 | 05/01/21 | 04/30/30 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 46,274 | 23,139 |
| 05/04/21 | 73.73 | 05/04/22 | 05/03/31 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17,799 | 35,599 |
| 05/03/22 | 87.10 | 05/03/23 | 05/02/32 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 58,783 |
| 05/02/23 | 117.89 | 05/02/24 | 05/01/33 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| 46,922 | (3) | 5,115,436 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| 55,926 | (4) | 6,097,053 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | 66,913 |
|
| 05/03/19 | 77.62 | 05/03/20 | 05/02/29 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 87,170 |
|
| 05/01/20 | 75.36 | 05/01/21 | 04/30/30 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 58,894 | 29,449 |
| 05/04/21 | 73.73 | 05/04/22 | 05/03/31 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19,417 | 38,835 |
| 05/03/22 | 87.10 | 05/03/23 | 05/02/32 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 45,643 |
| 05/02/23 | 117.89 | 05/02/24 | 05/01/33 |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| 51,188 | (3) | 5,580,516 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| 43,426 | (4) | 4,734,303 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Merck & Co., Inc. 20232024 Proxy Statement
| 76 |
ç ç ç |
Outstanding Equity Awards |
Outstanding Equity Awards for Fiscal Year Ended December 31, 20222023
| Option Awards | Stock Awards | Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Name | Number of Securities Underlying Unexercised Options Exercisable (#)(1) | Number of Securities Underlying Unexercised Options Unexercisable (#)(1) | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Grant Date | Option Exercise Price ($) | Vesting Date | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#)(2) | Market Value of Shares or Units of Stock That Have Not Vested ($)(2)(5) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(5) | Number of | Number of | Equity | Grant | Option | Vesting | Option | Number | Market | Equity | Equity | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Guindo | 110,705 | $12,282,720 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DeLuca | 58,185 | 05/03/19 | $77.62 | 05/03/20 | 05/02/29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0 | (3) | $0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| 57,064 | (4) | $6,331,251 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 77,830 | 05/01/20 | 75.36 | 05/01/21 | 04/30/30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 56,792 | 28,396 | 05/04/21 | 73.73 | 05/04/22 | 05/03/31 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19,417 | 38,835 | 05/03/22 | 87.10 | 05/03/23 | 05/02/32 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 44,260 | 05/02/23 | 117.89 | 05/02/24 | 05/01/33 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 27,126 | $2,957,277 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 51,188 | (3) | $5,580,516 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| 42,110 | (4) | 4,590,832 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Li | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Li | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Li | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Li | 14,702 | 05/05/17 | 62.07 | 05/05/18 | 05/04/27 | 14,702 | 05/05/17 | 62.07 | 05/05/18 | 05/04/27 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15,087 | 05/04/18 | 56.04 | 05/04/19 | 05/03/28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17,455 | 05/03/19 | 77.62 | 05/03/20 | 05/02/29 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15,087 | 05/04/18 | 56.04 | 05/04/19 | 05/03/28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14,008 | 7,006 | 05/01/20 | 75.36 | 05/01/21 | 04/30/30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2,592 | 1,299 | 05/01/20 | 75.36 | 05/01/21 | 04/30/30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 31,550 | 63,104 | 05/04/21 | 73.73 | 05/04/22 | 05/03/31 | 17,455 | 05/03/19 | 77.62 | 05/03/20 | 05/02/29 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 75,728 | 05/03/22 | 87.10 | 05/03/23 | 05/02/32 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 896 | $99,411 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 21,014 | 05/01/20 | 75.36 | 05/01/21 | 04/30/30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 166 | $18,417 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 56,154 | (3) | $6,230,286 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| 66,544 | (4) | $7,383,056 | 3,891 | 05/01/20 | 75.36 | 05/01/21 | 04/30/30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Zachary | 75,436 | 05/04/18 | 56.04 | 05/04/19 | 05/03/28 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25,946 | 05/01/20 | 75.36 | 05/01/21 | 04/30/30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 56,792 | 05/04/21 | 73.73 | 05/04/22 | 05/03/31 | 63,100 | 31,554 | 05/04/21 | 73.73 | 05/04/22 | 05/03/31 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 58,252 | 05/03/22 | 87.10 | 05/03/23 | 05/02/32 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50,538 | (3) | $5,607,191 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25,242 | 50,486 | 05/03/22 | 87.10 | 05/03/23 | 05/02/32 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| 51,188 | (4) | $5,679,308 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 77,455 | 05/02/23 | 117.89 | 05/02/24 | 05/01/33 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 66,544 | (3) | 7,254,627 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| 73,692 | (4) | 8,033,902 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (1) | Stock options generally vest and become exercisable in equal installments on the first, second and third anniversaries of the grant date, and expire on the day before the tenth anniversary of the grant date. The date set forth in the “Vesting Date” column represents the first vesting date for such award. |
| (2) |
|
|
DeLuca’s continued employment. The RSUs are payable in shares of Merck common |
| (3) |
|
Maximum (200% of target) of PSUs granted during 2022 that may be earned based on Merck’s performance, as determined by the C&MD Committee, following the completion of the three-year performance period ending December 31, 2024. |
| (4) | Maximum (200% of target) of PSUs granted during 2023 that may be earned based on Merck’s performance, as determined by the C&MD Committee, following the completion of the three-year performance period ending December 31, 2025. |
| (5) | The market value of the units reported in this column was computed by multiplying the number of such units by |
Merck & Co., Inc. 20232024 Proxy Statement
|
|
|
|
|
|
Merck & Co., Inc. 2023 Proxy Statement
|
| ÷ ÷ ÷ ÷ | 77 |
Option Exercises and Stock Vested
The following table provides information about stock options that were exercised and stock units that vested during 2022.2023.
Option Exercises and Stock Vested for Fiscal Year Ended December 31, 20222023
| Option Awards | Stock Awards | Option Awards | Stock Awards | |||||||||||||||||||||||||||||
Name | Number of Shares Acquired on Exercise (#) | Value Realized on Exercise ($)(1) | Number of Shares Acquired on Vesting (#)(2) | Value Realized on Vesting ($)(3) | Number of Shares Acquired on Exercise (#) | Value Realized on Exercise ($)(1) | Number of Shares Acquired on Vesting (#)(2) | Value Realized on Vesting ($)(3) | ||||||||||||||||||||||||
Davis | 167,613 | $6,766,101 | 44,233 | (a) | $4,860,322 | |||||||||||||||||||||||||||
Frazier | 2,172,853 | 95,766,852 | 174,168 | (a) | 19,137,580 | |||||||||||||||||||||||||||
Davis | ||||||||||||||||||||||||||||||||
Davis | ||||||||||||||||||||||||||||||||
Davis | 143,329 | $8,440,688 | 148,374 | (a) | $17,720,307 | |||||||||||||||||||||||||||
Litchfield | — | — | 5,762 | (a)(b) | 597,594 | |||||||||||||||||||||||||||
Litchfield | ||||||||||||||||||||||||||||||||
Litchfield | ||||||||||||||||||||||||||||||||
Litchfield | — | — | 36,347 | (a)(b) | 4,337,493 | |||||||||||||||||||||||||||
Guindo | — | — | — | (a) | — | |||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||
Chattopadhyay | 218,049 | 12,488,854 | 45,157 | (a) | 5,393,101 | |||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||
DeLuca | 141,599 | 8,027,422 | 43,544 | (a) | 5,200,460 | |||||||||||||||||||||||||||
Li | — | — | 6,890 | (a)(b) | 717,407 | |||||||||||||||||||||||||||
Zachary | 148,647 | 3,461,623 | 27,646 | (a) | 3,037,742 | |||||||||||||||||||||||||||
Li | ||||||||||||||||||||||||||||||||
Li | ||||||||||||||||||||||||||||||||
Li | — | — | 49,446 | (a)(b) | 5,901,130 | |||||||||||||||||||||||||||
| (1) | This column represents the values realized upon stock option exercises during |
| (2) | This column represents the vesting during |
| (a) | PSUs granted in |
| (b) | In addition to the |
| (3) | The value realized for PSUs was determined by multiplying the number of vested units by the market price of Merck common stock on January |
Merck & Co., Inc. 20232024 Proxy Statement
| 78 | ç ç ç ç |
|
Pension Benefits
The table below provides information concerning the present value of benefits accumulated by the Named Executive Officers from the Merck U.S. Pension Plan (the “Qualified Plan”), MSD Supplemental Retirement Plan (the “SRP”), and U.K. Pension Plan (see footnote 54 below). The terms of the Qualified Plan and SRP are described below.
Pension Benefits for Fiscal Year Ended December 31, 20222023
Name | Plan Name | Number of Years Credited Service (#)(1) | Number of Years Cash Balance Service (#)(2) | Present Value of Accumulated Benefit ($)(3) | Payments During Last Fiscal Year ($) | |||||||||||||||
Davis | Qualified Plan | — | 8.67 | $220,080 | $0 | |||||||||||||||
|
| SRP | — | 8.67 | 1,636,803 | 0 | |||||||||||||||
Frazier(4) | Qualified Plan | 27.50 | 30.50 | 1,376,726 | 0 | |||||||||||||||
|
| SRP | 27.50 | 30.50 | 25,451,129 | 0 | |||||||||||||||
Litchfield(5) | Qualified Plan | — | 32.25 | 97,960 | 0 | |||||||||||||||
| SRP | — | 32.25 | 370,259 | 0 | ||||||||||||||||
|
| U.K. Pension Plan | 26.25 | — | 2,190,337 | 0 | |||||||||||||||
Guindo(6) | Qualified Plan | 26.83 | 27.83 | 1,128,145 | 0 | |||||||||||||||
|
| SRP | 26.83 | 27.83 | 4,882 | 1,067,839 | |||||||||||||||
Li | Qualified Plan | — | 5.75 | 154,212 | 0 | |||||||||||||||
|
| SRP | — | 5.75 | 476,790 | 0 | |||||||||||||||
Zachary | Qualified Plan | — | 4.67 | 93,179 | 0 | |||||||||||||||
|
| SRP | — | 4.67 | 410,733 | 0 | |||||||||||||||
Name | Plan Name | Number of Years Credited Service (#)(1) | Number of Years Cash Balance Service (#)(2) | Present Value of Accumulated Benefit ($)(3) | Payments During Last Fiscal Year ($) | |||||||||||||||
Davis | Qualified Plan | — | 9.67 | $266,289 | $0 | |||||||||||||||
|
| SRP | — | 9.67 | 2,241,757 | 0 | |||||||||||||||
Litchfield(4) | Qualified Plan | — | 33.25 | 141,103 | 0 | |||||||||||||||
| SRP | — | 33.25 | 663,386 | 0 | ||||||||||||||||
|
| U.K. Pension Plan | 26.25 | — | 2,646,601 | 0 | |||||||||||||||
Chattopadhyay | Qualified Plan | 10.00 | 14.08 | 621,463 | 0 | |||||||||||||||
|
| SRP | 10.00 | 14.08 | 2,847,348 | 0 | |||||||||||||||
DeLuca | Qualified Plan | 8.00 | 12.25 | 518,902 | 0 | |||||||||||||||
|
| SRP | 8.00 | 12.25 | 2,484,870 | 0 | |||||||||||||||
Li | Qualified Plan | — | 6.75 | 192,053 | 0 | |||||||||||||||
|
| SRP | — | 6.75 | 778,198 | 0 | |||||||||||||||
| (1) | This column shows the number of years of Credited Service that is used for benefit accrual purposes and eligibility purposes under the Final Average Pay formula of the Qualified Plan and the SRP. The Final Average Pay formula is applicable only for participants who were actively employed on December 31, 2012. Participants hired (or rehired) after December 31, 2012 receive benefits under a Cash Balance formula that does not rely on Credited Service. |
For employees actively employed on December 31, 2012, Credited Service for the Final Average Pay formula begins with the January 1 or July 1 that coincides with or follows a participant’s hire date and ends with the last full month of employment. Credited Service is earned through the earlier of termination or December 31, 2019. After December 31, 2019, all benefits will be calculated under the Cash Balance formula. A maximum of 35 years of Credited Service may be earned. Mr. |
The number of years of Credited Service for Ms. Litchfield in the U.K. Pension Plan row shown is used for benefit accrual purposes while she participated in the plan prior to her transfer from the U.K. to the U.S. |
| (2) | This column shows the number of years of Cash Balance Service that is used for benefit accrual purposes under the Cash Balance formula of the Qualified Plan and the SRP. |
Cash Balance Service begins on a participant’s first day of employment, includes all years and completed months of service, and ends on the participant’s date of termination of employment. |
The Cash Balance Service for Ms. Litchfield is based on her original hire date and is used to determine the pay credit level under the Cash Balance formula. Benefit accruals under the Cash Balance formula did not start until January 1, 2020, effective with her transfer to the U.S. Prior to January 1, 2020, Ms. Litchfield was not a U.S. based employee and, therefore, not eligible for the Qualified Plan and SRP. |
| (3) | For the Qualified Plan and the SRP, the actuarial present value is calculated using the same assumptions used for financial statement reporting purposes as set forth in the footnotes to our financial statements, except that commencement is assumed at the earliest unreduced retirement age (with no pre-retirement mortality). The earliest unreduced retirement age is the earlier of age 62 and 10 years of Credited Service (including service under the Cash Balance formula) or age 65 with no service requirement. As of December 31, |
Discount rate equals 5.50%5.25% for the Qualified Plan and 5.80%5.38% for the SRP;
Interest crediting rate equals 5.35% for both the Qualified Plan and the SRP;
Merck & Co., Inc. 20232024 Proxy Statement
Pension Benefits | ÷ ÷ ÷ ÷ | 79 |
Interest crediting rate equals 5.30% for both the Qualified Plan and the SRP;
| • | Mortality based on 100% of the sex distinct Pri-2012 White Collar Mortality Table, with projection based on modified MP-2021 Projection Scale using a 0.75% ultimate rate at most ages; |
Future lump sum conversion factors calculated by implied forward rates embedded in the 12/31/20222023 Willis Towers Watson RATE:Link 60th to 90th percentile yield curve and mortality defined in Internal Revenue Code Section 417(e)(3); and
Assumes that 80% of retirees elect a lump sum and the remaining 20% elect an annuity for the Qualified Plan and assumes 100% of retirees elect a lump sum for the SRP.
| (4) |
|
The amounts in the table for the U.K. Pension Plan for Ms. Litchfield reflect benefits accrued during her participation in the plan plus legally required U.K. pension consumer price index increases. The amounts reported represent the actuarial present value of the accrued benefit payable at age 65 and converted from GBP to USD using an exchange rate ($1/£) of |
|
The NEOs participate in both U.S. defined benefit plans, as do other U.S.-based Merck Sharp & Dohme LLC salaried employees. Benefits payable under the Qualified Plan and the SRP are based on several formulas.
Beginning in 2013, a Cash Balance formula was added to replace the Final Average Pay (“FAP”) formula. Employees eligible for U.S. benefits on December 31, 2012 receive transition benefits, which provide the greater of the benefit under the Cash Balance and FAP formulas through December 31, 2019, or the date the participant terminates employment or loses retirement plan eligibility, if earlier. Only Mr. Guindo isDeLuca and Mr. Chattopadhyay are eligible for transition benefits.
Final Average Pay Formula: For service prior to January 1, 2013, benefits are calculated and shown as a single life annuity normally payable at age 65 (normal retirement date or “NRD”). The amount equals:

* Limited to 31.25
Final Average Pay. The average of a participant’s highest five consecutive calendar years of Total Pay for the 10 years before the earlier of:
Termination of employment, or
December 31, 2019, if eligible for the transition provisions.
Cash Balance Formula: For service starting January 1, 2013, benefits are calculated and shown as an account balance that grows with annual pay credits according to the following schedule:
Age + Cash Balance Service at 12/31 | Percent of Total Pay credited to Account Balance | |||
| At Least | Less Than | |||
| — | 40 | 4.5% | ||
| 40 | 50 | 5.5% | ||
| 50 | 60 | 6.5% | ||
| 60 | 70 | 8.0% | ||
| 70 | — | 10.0% | ||
The account balance also earns interest credits every year at the annual rate of the change in the Consumer Price Index plus 3% (and not less than 3.3%). The account balance is the sum of annual pay credits and interest credits.
Final Average Pay. The average of a participant’s highest five consecutive calendar years of Total Pay for the 10 years before the earlier of:
Termination of employment, or
December 31, 2019, if eligible for the transition provisions.
Total Pay is generally base salary and EIP for both the FAP and Cash Balance formulas for the NEOs.
Merck & Co., Inc. 2023 Proxy Statement
|
|
Vesting. A participant is generally vested after three years of vesting service. All NEOs are vested. A participant who is vested and terminates employment can commence receiving a reduced pension benefit after attaining age 55. FAP benefits are reduced on an actuarial basis. Participants who only have vested benefits under the Cash Balance formula can commence payment of their Qualified Plan benefit immediately upon termination.
Merck & Co., Inc. 2024 Proxy Statement
| 80 | ç ç ç ç | Pension Benefits |
Early Retirement Subsidies. Under the FAP formula, a participant who is age 55 with at least 10 years of credited service is entitled to early retirement subsidies. Under this provision, unreduced benefits may begin at age 62 and benefits that begin before 62 are only reduced by 3% per year. As of December 31, 2023, Mr. Chattopadhyay, and Mr. DeLuca are retirement eligible under the Qualified Plan and the SRP (i.e., at least age 55 and having completed at least 10 years of Credited Service).
SRP Benefits. The Qualified Plan benefits are limited by the Internal Revenue Code. The SRP is an unfunded plan maintained to provide benefits according to the formulas described above without regard to those limits. The SRP also may include benefits based on compensation deferred into the Merck Deferral Program.
Forms of Benefit. In the Qualified Plan and in the SRP for accruals prior to 2005, a participant generally can choose from several annuity options or a lump sum. SRP accruals post-2004 are payable in a lump sum or installments of 5 to 10 years. All forms of benefit are actuarially equivalent to the single life annuity.
Merck & Co., Inc. 20232024 Proxy Statement
| ÷ ÷ ÷ ÷ | 81 |
Nonqualified Deferred Compensation
The following table shows the executive contributions, earnings, and account balances for the Named Executive Officers in the Merck Deferral Program, an unfunded, nonqualified, unsecured deferred compensation plan. The Merck Deferral Program allows participants who are executive officers to defer all or a portion of annual bonus and/or up to 50% of base salary, subject to certain limitations.
Nonqualified Deferred Compensation for Fiscal Year Ended December 31, 20222023
Name | Executive in 2022 ($) | Registrant in 2022 ($)(1) | Aggregate Earnings in 2022 ($)(2) | Aggregate Withdrawals/ in 2022 ($) | Aggregate Balance at 12/31/22 ($)(3) | Executive Contributions in 2023 ($) | Registrant Contributions in 2023 ($)(1) | Aggregate Earnings in 2023 ($)(2) | Aggregate Withdrawals/ Distributions in 2023 ($) | Aggregate Balance at 12/31/23 ($)(3) | ||||||||||||||||||||||||||||||
Davis | $0 | $182,890 | $(197,887 | ) | $0 | $1,062,135 | ||||||||||||||||||||||||||||||||||
Frazier | 0 | 163,950 | (4,353,982 | ) | 0 | 21,675,777 | ||||||||||||||||||||||||||||||||||
Davis | ||||||||||||||||||||||||||||||||||||||||
Davis | ||||||||||||||||||||||||||||||||||||||||
Davis | $0 | $242,730 | $213,020 | $0 | $1,517,885 | |||||||||||||||||||||||||||||||||||
Litchfield | 0 | 82,660 | (21,400 | ) | 0 | 155,228 | ||||||||||||||||||||||||||||||||||
Litchfield | ||||||||||||||||||||||||||||||||||||||||
Litchfield | ||||||||||||||||||||||||||||||||||||||||
Litchfield | 0 | 112,315 | 42,976 | 0 | 310,519 | |||||||||||||||||||||||||||||||||||
Guindo | 0 | 2,146 | (4 | ) | 0 | 2,142 | ||||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | ||||||||||||||||||||||||||||||||||||||||
Chattopadhyay | 0 | 99,300 | 700,674 | 0 | 11,068,547 | |||||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||||||||||
DeLuca | ||||||||||||||||||||||||||||||||||||||||
DeLuca | 226,950 | 94,081 | 937,585 | 0 | 4,877,634 | |||||||||||||||||||||||||||||||||||
Li | 0 | 98,508 | (47,283 | ) | 0 | 278,423 | ||||||||||||||||||||||||||||||||||
Zachary | 96,815 | 89,715 | (67,806 | ) | 0 | 444,572 | ||||||||||||||||||||||||||||||||||
Li | ||||||||||||||||||||||||||||||||||||||||
Li | ||||||||||||||||||||||||||||||||||||||||
Li | 0 | 146,717 | 100,553 | 0 | 525,693 | |||||||||||||||||||||||||||||||||||
| (1) | The amounts disclosed in this column represent the Company’s annual 4.5% credit of eligible pay in excess of the IRS limit to the NEOs’ accounts under the Merck Deferral Program. These amounts are included within the amount disclosed in the “All Other Compensation” column of the Summary Compensation table for each applicable NEO for |
| (2) | This column represents dividends earned plus changes in market and account value (investment earnings or losses) for the period of January 1, |
| (3) | This column includes deferred compensation earned in earlier years which was disclosed as “Salary,” “Non-Equity Incentive Plan Compensation” or “All Other Compensation” in the Summary Compensation table of prior proxy statements as follows: Mr. Davis, $182,890 for 2022 and $92,124 for |
Merck Deferral Program Investments. Account balances may be invested in phantom investments selected by the executive from an array of investment options that mirrors the funds in the Merck U.S. Savings Plan.
Distributions. When participants elect to defer amounts into the Merck Deferral Program, they also elect when the amounts ultimately will be distributed to them. Distributions may either be made in a specific year (regardless of whether employment has then ended) or at a time that begins at or after the executive’s employment has ended. Distributions can be made in a lump sum or up to 15 annual installments. Distributions from the Merck common stock fund are made in shares, with cash payable for any partial share.
Merck & Co., Inc. 20232024 Proxy Statement
| 82 | ç ç ç ç |
|
Potential Payments Upon
Termination or a Change in Control
The section below describes the payments and benefits that may be made to the NEOs upon termination from employment, including in connection with a change in control (as defined below). For payments that may be made to the NEOs upon retirement, see the Pension Benefits table and related narrative beginning on page 77.78.
Merck & Co., Inc. U.S. Separation Benefits Plan, as amended (the “Separation Plan”)
The Separation Plan provides severance pay and benefits to certain eligible employees, including the NEOs, whose employment is terminated due to organizational changes, including discontinuance of operations, location closings, corporate restructuring, or a work force reduction. For the NEOs, the following severance pay and benefits are payable under the Separation Plan:
A cash lump sum severance payment, the amount which for the NEOs, is determined as follows:
Years of Continuous Service at Separation Date | Severance Pay (base salary in weeks) | |
Less than 1 year | 26 | |
1-4 years | 40 | |
5 years or more | 40 plus 2 additional weeks for each year of continuous service beyond 4 years (maximum 78 total) | |
Continued participation in the Company’s medical, dental, and basic life insurance plans at active-employee rates for 26 to 78 weeks (determined based on years of continuous service); and
Outplacement services for up to 12 months.
To be eligible for the severance pay and benefits described above, a general release of claims is required, as well as compliance with applicable restrictive covenants.
Effects of Termination on Other Awards or Under Other Benefit Plans
The NEOs may be eligible for additional payments and benefits upon termination from employment as described below.
| • | EIP Awards. The NEOs may be eligible for payments in lieu of EIP bonus payouts as follows: |
Timing of Termination | Eligibility for Pay in Lieu of EIP | Determination of Amount of Pay in Lieu of EIP | ||
Termination occurs following the end of the performance year | Eligibility determined in accordance with same terms and conditions as other employees participating in the EIP | Amount determined in accordance with same terms and conditions as other employees participating in the EIP | ||
Termination occurs between January 1 and June 30 of the performance year | Eligible for special payment if NEO is retirement eligible | Amount of the special payment is based on the NEO’s target award and the number of months worked in the performance year | ||
Termination occurs after June 30 and before December 31 of the performance year | Eligible for special payment | Amount of the special payment is based on the NEO’s target award and the number of months worked in the performance year | ||
| • | Retirement Plan Bridge. NEOs who are age 50 or above with at least 10 years of Cash Balance Service as of December 31 of the year of separation may be eligible for a pro-rata portion of the early retirement subsidies described in the Pension Benefits section beginning on page |
Merck & Co., Inc. 20232024 Proxy Statement
Potential Payments Upon Termination or a Change in Control
| ÷ ÷ ÷ ÷ | 83 |
| • | Retiree Healthcare Bridge. NEOs who are age 50 or above with 10 years of Cash Balance Service as of December 31 of the year of separation may be eligible for subsidized retiree medical benefits in accordance with the plan provisions applicable to similarly situated retired employees, as |
Options, RSUs and PSUs
| • |
|
| • | Options: If an NEO retiree has unvested stock options, the portion of the applicable stock option that would have become vested |
| • | RSUs: If an NEO retiree has unvested RSUs, a |
| • | PSUs: On the regularly scheduled determination date set forth in the applicable award agreement, an NEO retiree will become vested in a pro-rata portion of the PSUs |
| • | Upon involuntary termination (other than in the case of a termination by reason of non-performance of duties, sale, death, disability, or |
| • | Options: Unvested stock options |
| • | RSUs: If the NEO has unvested RSUs, a pro rata portion of the unvested RSUs will vest and the underlying shares of Merck common stock and the applicable accrued dividend equivalents will be distributed to the NEO on the next scheduled vesting date following the NEO’s termination, so long as such termination occurs on or after the first anniversary of the grant date. The pro rata portion is determined based on the number of completed months of employment during the three-year vesting period relative to the total length of the period, i.e., 36 months, less any RSUs that had already vested under the applicable award. The remaining unvested portion of the award is forfeited as of the termination date. |
| • | PSUs: On the regularly scheduled determination date as set forth in the applicable award agreement, the NEO will become vested in a |
NEOs who are entitled to the Retirement Plan Bridge, as described above, are also eligible to be treated in accordance with the equity plan’s provisions applicable to retired employees with respect to stock options granted to them before 2013. For stock option, RSU and PSU grants occurring during or after 2013, generally, separated “bridged” employees are eligible to be treated in accordance with the equity plan’s provisions applicable to retired employees only if they are also eligible for subsidized retiree medical benefits. If the “bridged” employees are not also eligible for subsidized retiree medical benefits, separated bridged employees will be treated in accordance with the equity plan’s provisions applicable to involuntarily terminated employees, as described above.
Merck & Co., Inc. 2024 Proxy Statement
| 84 | ç ç ç ç | Potential Payments Upon Termination or a Change in Control Individual Agreements and Arrangements |
Individual Agreements and Arrangements
When Robert M. Davis was appointed Executive Vice President and Chief Financial Officer by the Board, effective April 23, 2014, to compensate Mr. Davis for pension benefits he forfeited upon leaving his prior employer, his offer letter provides for a cash payment of $2,000,000 within 90 days of his termination of employment (other than for cause) provided that he was employed for at least 10 years with no breaks in service. The terms of this offer letter continue in full force and effect following his promotions that occurred in 2021 and 2022.
On March 27, 2018, the Board appointed Jennifer Zachary as Executive Vice President and General Counsel, effective April 16, 2018. The Company’s offer letter, dated March 16, 2018, provides that Ms. Zachary is eligible to receive enhanced severance in the event of termination for reason other than cause or if terminated for good reason within 24 months of the appointment of a successor CEO to Kenneth Frazier equal to one times base salary plus target annual cash incentive.
When Chirfi Guindo was appointed as Chief Marketing Officer, Human Health, effective July 1, 2022, the Company’s offer letter, dated June 8, 2022, provided for Mr. Guindo to receive the following: (a) annual base salary of $700,000, subject to annual review by the C&MD Committee; (b) eligibility to participate in the Company’s EIP with a target bonus for the 2022 performance year of 80% of his annual base salary; (c) eligibility to receive an annual equity-based grant, beginning in 2023, pursuant to the Company’s LTI program with a target value of $2,500,000; (d) sign-on cash award of $530,000, payable within 30 days following his start date; (e) sign-on equity award of $12,200,000, of which $2,500,000 was provided in the form of a 2022 PSU grant and $9,700,000 was provided in the form of RSUs, and the majority of which was provided to buy out forfeited equity at his prior company; (f) in addition to standard separation benefits, continued vesting of his sign-on equity award (including the RSUs and PSUs) in the event of a termination for reasons other than cause or if terminated for good reason, in each case if such termination occurs prior to when all of his sign-on equity is fully vested; (g) reimbursement of certain expenses associated with Mr. Guindo’s move of his residence, in accordance with the Company’s Relocation Program; and (h) participation in other benefits plans in accordance with the Company’s practices for other executives of a similar level, including participation in the Company’s Change in Control Plan. The grants constituting Mr. Guindo’s sign-on equity award were made on the first quarterly grant date immediately following his start date. The PSUs will vest at the end of the 2022 – 2024 performance period and are subject to the performance of the 2022 PSU program. The RSUs will vest in equal installments on the first, second, and third anniversaries of the grant date.
Merck & Co., Inc. 2023 Proxy Statement
|
|
As part of the transition from his roles with the Company, Mr. Frazier will be provided with access to office space, within existing Company facilities, and part-time administrative support services, for a period of 7 years following his retirement on November 30, 2022. The estimated incremental annual cost of this benefit is approximately $125,000. Mr. Frazier is responsible for the payment of any taxes that may result from the provision of these benefits. In addition, following his retirement, Mr. Frazier no longer uses Company aircraft or a Company car or driver for personal travel.
Effects of Change in Control under Change in Control, Equity, and Other Benefit Plans
Merck & Co., Inc. Change in Control Separation Benefits Plan, as amended (the “Change in Control Plan”)
The Change in Control Plan provides for the following severance payments and benefits upon a termination without “cause,” or a resignation by the NEO for “good reason,” in each case within two years following a change in control:
Cash severance paid in a lump sum within 90 days following employment termination in an amount equal to three (for the CEO) or two (for the other NEOs) times the sum of (x) the NEO’s base salary, plus (y) the lesser of (a) the NEO’s target bonus amount or (b) the average of the actual bonuses paid to the NEO in the three years immediately preceding termination while the NEO was serving in the same position as he or she was serving immediately prior to termination, annualized for partial or incomplete years in such position;
| • | Pro-rata annual cash incentive at target levels, paid in a lump sum within 90 days following employment termination; |
Continued medical, dental and life insurance benefits at active-employee rates for a period of up to three years for the CEO and for up to two years for the other NEOs, which are reduced by benefits obtained from a subsequent employer;
If the NEO would have attained specified age and service levels within two years following the change in control, then the NEO is entitled to (1)(a) subsidized and/ or unreduced pension benefits upon commencement of pension benefits in accordance with plan terms after termination of employment, and (2)(b) subsidized retiree medical benefits as a retiree under our health plans commencing immediately after the expiration of the benefit continuation period at active-employee rates as described above;
An NEO who is a participant in the Company’s pension plan will become vested (if not already) in the applicable accrued benefit as of the termination date;
Continued financial planning benefits for up to 12 months; and
Outplacement services for a period of time as provided under the Separation Plan.
An NEO is not eligible for benefits under the Change in Control Plan following a termination due to death or permanent disability.
For purposes of the Change in Control Plan:
A “change in control” generally consists of:
| (i) | an acquisition of more than 30% of the Company’s voting securities (other than acquisitions directly from the Company); |
| (ii) | the current Board (and their approved successors) ceasing, over any consecutive 24-month period, to constitute a majority of the board of directors of a successor to the Company; |
| (iii) | the consummation of a merger, consolidation or reorganization, unless (a) the shareholders of the Company prior to the transaction hold at least 50% of the voting securities of the successor, (b) the members of the Board prior to the transaction constitute at least a majority of the board of directors of the successor, and (c) no person owns 30% or more of the voting securities of the Company or the successor; or |
| (iv) | shareholder approval of the liquidation or dissolution of the Company or the sale by the Company of all or substantially all of its assets. |
A termination for “good reason” generally includes any of the following actions without the executive’s written consent:
| (i) | significantly and adversely changing the executive’s authority, duties, responsibilities or position (including title or reporting level) other than: |
| (a) | an isolated, insubstantial and inadvertent action not taken in bad faith that the Company remedies promptly after receiving notice; |
Merck & Co., Inc. 2024 Proxy Statement
Potential Payments Upon Termination or a Change in Control Effects of Change in Control under Change in Control, Equity, and Other Benefit Plans | ÷ ÷ ÷ ÷ | 85 |
| (b) | a change in the person to whom (but not the position to which) the NEO reports; |
| (c) | ceasing to be an executive officer subject to Section 16(b) of the Exchange Act; or |
| (d) | transfer of employment to an affiliate of the Company, if such transfer occurs prior to a change in control; |
Merck & Co., Inc. 2023 Proxy Statement
|
|
| (ii) | reducing annual base salary or level of bonus opportunity; |
| (iii) | changing the executive’s office location so the executive must commute more than the greater of (a) 50 more miles or (b) 120% more miles, as compared to his or her commute immediately prior to the change; |
| (iv) | failing to pay base salary, bonus or deferred compensation under any Company deferred compensation program within seven days of its due date; |
| (v) | failing to continue any material compensation plan or program in which the executive participates, including bonus plans and the Incentive Stock Plan (or successors to those plans), or failing to continue the executive’s level of participation in those plans; |
| (vi) | failing to continue to provide the executive with pension and welfare benefits substantially similar to those in which he or she participates, or materially reducing any of those benefits or depriving the executive of any material fringe benefit; |
| (vii) | failing to obtain a satisfactory agreement from any successor to Merck to assume and agree to perform the obligations under the Change in Control Plan; and |
| (viii) | any purported termination of the executive’s employment by the Company or its subsidiaries that is not properly effected pursuant to the notice provisions of the Change in Control Plan. |
A termination by the Company for “Cause” generally includes:
willful and continued failure by the executive to substantially perform his or her duties for the Company (other than any failure that results from incapacity due to physical or mental illness or any such actual or anticipated failure after the issuance of a notice of termination for good reason) for at least 30 consecutive days after a written demand for substantial performance has been delivered;
willful misconduct or gross negligence by the executive that is demonstrably and materially injurious to the Company or any of its subsidiaries; or
| (i) | willful and continued failure by the executive to substantially perform his or her duties for the Company (other than any failure that results from incapacity due to physical or mental illness or any such actual or anticipated failure after the issuance of a notice of termination for good reason) for at least 30 consecutive days after a written demand for substantial performance has been delivered; |
| (ii) | willful misconduct or gross negligence by the executive that is demonstrably and materially injurious to the Company or any of its subsidiaries; or |
conviction, or entry of a plea of nolo contendere, to a felony or any crime (whether or not a felony) involving dishonesty, fraud, embezzlement or breach of trust.
| (iii) | conviction, or entry of a plea of nolo contendere, to a felony or any crime (whether or not a felony) involving dishonesty, fraud, embezzlement or breach of trust. |
To be eligible for the severance pay and benefits described above, a general release of claims is required, as well as compliance with applicable restrictive covenants. The severance benefits provided under the Change in Control Plan are in lieu of (or offset by) any other severance benefits to which an NEO may be entitled under other arrangements.
The NEOs are not entitled to any tax gross-up in the event they are subject to excise taxes payable under Section 4999 of the Internal Revenue Code in connection with a change in control.
Treatment of Equity Upon a Change in Control under the Merck & Co., Inc. 2010 Stock Incentive Plan and Merck & Co., Inc. 2019 Stock Incentive Plan (the “Equity Plans”)
The Equity Plans and applicable equity award terms and conditions provide for the following treatment of stock options, RSUs and PSUs upon termination of employment and/or a change in control:
In general, stock options that become vested may be exercised for five years following termination of the option holder’s employment following a change in control (but not beyond the original term of the stock option). This extended exercise period would not apply in the case of a termination by reason of death or retirement or for gross misconduct.
If stock options do not remain outstanding following the change in control and are not converted into successor stock options, then option holders will be entitled to receive cash for each stock option in an amount equal to the difference between the price paid to shareholders in the change in control and the applicable exercise price.
Effects of Change in Control under Other Benefit Plans
Our compensation and employee benefit plans, programs and arrangements generally provide that for two years following the change in control, the material terms of the plans, programs and arrangements (including terms relating to eligibility, benefit calculation, benefit accrual, cost to participants, subsidies and rates of employee contributions) may not be modified in a manner that is materially adverse to individuals who participated in them immediately before the change in control.
Merck & Co., Inc. 2024 Proxy Statement
| 86 | ç ç ç ç | Potential Payments Upon Termination or a Change in Control Effects of Change in Control under Change in Control, Equity, and Other Benefit Plans |
The following table estimates the dollar value of the payments and benefits the NEOs would have been entitled to receive under the applicable plans and arrangements described above, assuming termination from employment occurred on December 31, 2022,2023, including in connection with a change in control. As of December 31, 2022,2023, Ms. Litchfield, is Retiree Healthcare BridgeMr. Chattopadhyay and Mr. DeLuca are retirement eligible (i.e., at least age 50 and having completed at least 10 years of Cash Balance Service) and Mr. Guindo is retirement eligible (i.e. at least age 55 and having completed at least 10 years of Credited Service). As of December 31, 2023, Mr. Guindo’sDavis would be retirement eligibility reflects his tenure with Merck, prior to his most recent hire date.eligible within two years of a change in control and therefore would be eligible for subsidized retiree medical benefits.
Name | Type of Payment or Benefit | Involuntary Termination | Change in Control | Involuntary Termination | ||||||||||
Davis | Severance Pay(1) | $1,552,885 | — | $12,112,500 | ||||||||||
| Supplemental Pension and Retiree Medical(2) | — | — | 92,468 | |||||||||||
| Welfare Benefits Continuation | 24,755 | — | 73,177 | |||||||||||
| Stock Option Accelerated Vesting(3) | — | — | 1,667,060 | |||||||||||
| PSU Accelerated Vesting(4) | — | — | 5,104,636 | |||||||||||
| RSU Accelerated Vesting | — | — | — | |||||||||||
| Outplacement, Financial Planning | 4,650 | — | 14,650 | |||||||||||
|
| TOTAL | $1,582,290 | — | $19,064,490 | ||||||||||
Litchfield | Severance Pay(1) | $1,687,500 | — | $4,500,000 | ||||||||||
| Supplemental Pension and Retiree Medical(2) | — | — | — | |||||||||||
| Welfare Benefits Continuation | 35,694 | — | 47,592 | |||||||||||
| Stock Option Accelerated Vesting(3) | — | — | 390,165 | |||||||||||
| PSU Accelerated Vesting(4) | — | — | 1,607,009 | |||||||||||
| RSU Accelerated Vesting | — | — | — | |||||||||||
| Outplacement, Financial Planning | 4,650 | — | 14,650 | |||||||||||
|
| TOTAL | $1,727,844 | — | $6,559,416 | ||||||||||
Chattopadhyay | Severance Pay(1) | $1,086,699 | — | $3,767,224 | ||||||||||
| Supplemental Pension and Retiree Medical(2) | — | — | — | |||||||||||
| Welfare Benefits Continuation | 14,053 | — | 28,106 | |||||||||||
| Stock Option Accelerated Vesting(3) | — | — | 425,632 | |||||||||||
| PSU Accelerated Vesting(4) | — | — | 1,247,827 | |||||||||||
| RSU Accelerated Vesting | — | — | — | |||||||||||
| Outplacement, Financial Planning | 4,650 | — | 14,650 | |||||||||||
|
| TOTAL | $1,105,402 | — | $5,483,438 | ||||||||||
DeLuca | Severance Pay(1) | $996,154 | — | $3,700,000 | ||||||||||
| Supplemental Pension and Retiree Medical(2) | — | — | — | |||||||||||
| Welfare Benefits Continuation | 19,521 | — | 39,042 | |||||||||||
| Stock Option Accelerated Vesting(3) | — | — | 425,632 | |||||||||||
| PSU Accelerated Vesting(4) | — | — | 1,210,012 | |||||||||||
| RSU Accelerated Vesting(4) | — | — | 410,733 | |||||||||||
| Outplacement, Financial Planning | 4,650 | — | 14,650 | |||||||||||
|
| TOTAL | $1,020,325 | — | $5,800,068 | ||||||||||
Li | Severance Pay(1) | $1,184,615 | — | $5,600,000 | ||||||||||
| Supplemental Pension and Retiree Medical(2) | — | — | — | |||||||||||
| Welfare Benefits Continuation | 26,821 | — | 71,522 | |||||||||||
| Stock Option Accelerated Vesting(3) | — | — | 2,220,194 | |||||||||||
| PSU Accelerated Vesting(4) | 4,240,274 | — | 7,644,264 | |||||||||||
| RSU Accelerated Vesting(4) | — | — | — | |||||||||||
| Outplacement, Financial Planning | 4,650 | — | 14,650 | |||||||||||
|
| TOTAL | $3,590,703 | — | $13,904,570 | ||||||||||
| (1) | Amounts included under “Involuntary Termination Before Change in Control” are based on the severance benefits payable to each named executive officer under the Separation Plan. Amounts included under “Involuntary Termination After Change in Control” are based on the severance benefits payable to each named executive officer under the Change in Control Plan. Amounts do not include any discretionary special payment in lieu of the EIP bonus payout. |
Merck & Co., Inc. 20232024 Proxy Statement
Potential Payments Upon Termination or a Change in Control Effects of Change in Control under Change in Control, Equity, and Other Benefit Plans |
÷ ÷ ÷ |
Name(5) | Type of Payment or Benefit | Involuntary Termination ($) | Change in Control ($) | Involuntary Termination ($) | ||||||||||
Davis | Severance Pay(1) | $1,426,154 | — | $11,587,500 | ||||||||||
| Supplemental Pension and Retiree Medical(2) | — | — | — | |||||||||||
| Welfare Benefits Continuation | 23,610 | — | 94,439 | |||||||||||
| Stock Option Accelerated Vesting(3) | — | — | 14,121,539 | |||||||||||
| PSU Accelerated Vesting(4) | 9,648,376 | — | 20,675,089 | |||||||||||
| RSU Accelerated Vesting | — | — | — | |||||||||||
| Outplacement, Financial Planning | 4,650 | — | 14,650 | |||||||||||
|
| TOTAL | $11,102,790 | — | $46,493,216 | ||||||||||
Litchfield | Severance Pay(1) | $1,462,500 | — | $3,900,000 | ||||||||||
| Supplemental Pension and Retiree Medical(2) | — | — | 125,697 | |||||||||||
| Welfare Benefits Continuation | 31,023 | — | 41,364 | |||||||||||
| Stock Option Accelerated Vesting(3) | 1,526,762 | — | 3,236,986 | |||||||||||
| PSU Accelerated Vesting(4) | 4,042,596 | — | 4,887,458 | |||||||||||
| RSU Accelerated Vesting | 56,806 | — | 96,083 | |||||||||||
| Outplacement, Financial Planning | 4,650 | — | 14,650 | |||||||||||
|
| TOTAL | $7,124,337 | — | $12,302,238 | ||||||||||
Guindo | Severance Pay(1) | $350,000 | — | $2,520,000 | ||||||||||
| Supplemental Pension and Retiree Medical(2) | — | — | — | |||||||||||
| Welfare Benefits Continuation | 9,836 | — | 39,342 | |||||||||||
| Stock Option Accelerated Vesting(3) | — | — | — | |||||||||||
| PSU Accelerated Vesting(4) | 6,331,251 | — | 3,165,625 | |||||||||||
| RSU Accelerated Vesting | 12,282,720 | — | 12,282,720 | |||||||||||
| Outplacement, Financial Planning | 4,650 | — | 14,650 | |||||||||||
|
| TOTAL | $18,978,457 | — | $18,022,337 | ||||||||||
Li | Severance Pay(1) | $1,009,615 | — | $5,000,000 | ||||||||||
| Supplemental Pension and Retiree Medical(2) | — | — | — | |||||||||||
| Welfare Benefits Continuation | 25,056 | — | 66,815 | |||||||||||
| Stock Option Accelerated Vesting(3) | — | — | 4,450,419 | |||||||||||
| PSU Accelerated Vesting(4) | 3,146,239 | — | 6,806,672 | |||||||||||
| RSU Accelerated Vesting(4) | 69,677 | — | 117,829 | |||||||||||
| Outplacement, Financial Planning | 4,650 | — | 14,650 | |||||||||||
|
| TOTAL | $4,255,237 | — | $16,456,384 | ||||||||||
Zachary | Severance Pay(1) | $749,771 | — | $3,801,339 | ||||||||||
| Supplemental Pension and Retiree Medical(2) | — | — | — | |||||||||||
| Welfare Benefits Continuation | 4,701 | — | 18,805 | |||||||||||
| Stock Option Accelerated Vesting(3) | — | — | 4,426,527 | |||||||||||
| PSU Accelerated Vesting(4) | 2,831,581 | — | 5,643,250 | |||||||||||
| RSU Accelerated Vesting(4) | — | — | — | |||||||||||
| Outplacement, Financial Planning | 4,650 | — | 14,650 | |||||||||||
|
| TOTAL | $3,590,703 | — | $13,904,570 | ||||||||||
|
| (2) | SRP enhancements include the incremental value of benefits provided upon a change in control. |
| (3) |
|
| (4) | The value equals the pro-rated or full total number of accelerated shares as of December 31, |
|
Merck & Co., Inc. 20232024 Proxy Statement
| 88 | | | | | |
| |||||||
Proposal 3
|
Pursuant to Rule 14A of the Securities and Exchange Act of 1934, as amended, we are providing our shareholders the opportunity to cast a non-binding, advisory vote on the frequency of future votes to approve the compensation of our Named Executive Officers as described in the Compensation Discussion and Analysis and compensation tables. Shareholders will be able to specify one of four choices for this proposal on the proxy card; one year, two years, three years or abstain. Shareholders are not voting to approve or disapprove the Board’s recommendation.
Our prior say on frequency vote occurred in 2017. At that year’s meeting, shareholders voted in support of annual advisory votes on future executive compensation proposals.
After careful consideration, the Board of Directors believes that it is appropriate and in the best interest for our shareholders to continue to cast a non-binding, advisory vote on executive compensation on an annual basis. The Board of Directors strongly believes in continuous, proactive engagement with its shareholders. Annual votes will facilitate this important goal by allowing management, the Board, and our shareholders to engage in a timely, open and meaningful dialogue and to receive input regarding the compensation of our Named Executive Officers, our corporate governance practices as well as our executive compensation program, policies and practices.
The shareholder vote on this resolution will not be binding on management or the Board of Directors and will not be construed as overruling any decision by management or the Board. Notwithstanding the Board’s recommendation and the outcome of the shareholder vote, the Board may in the future decide to conduct advisory votes on a more or less frequent basis and may vary its practice based on factors such as discussions with shareholders and the adoption of material changes to our executive compensation program.
|
Merck & Co., Inc. 2023 Proxy Statement
| ||||||
Ratification of Appointment of Independent Registered Public Accounting Firm for |
The Audit Committee is directly responsible for the appointment, compensation, retention and oversight of the Company’s independent registered public accounting firm. The Audit Committee has appointed PricewaterhouseCoopers LLP (“PwC”) to serve as our independent registered public accounting firm (the independent auditors) with respect to our operations for the year ending December 31, 2023,2024, subject to ratification by the holders of common stock of the Company. In taking this action, the Audit Committee considered carefully PwC’s performance in that capacity for the Company since its retention in 2002, its independence with respect to the services to be performed and its general reputation for adherence to professional auditing standards. The Audit Committee is responsible for the audit fee negotiations associated with the retention of PwC. The Audit Committee annually evaluates the performance of PwC, including the senior audit engagement team, and determines whether to reengage the independent registered public accounting firm.
The Audit Committee and the Board of Directors believe that the continued retention of PwC as our independent registered public accounting firm is in the best interests of the Company and our shareholders. Because the members of the Audit Committee value shareholders’ views on our independent auditors, a proposal for the ratification of the appointment of PwC will be presented at the 2024 Annual Meeting even though ratification is not legally required. If the appointment of PwC is not ratified, the matter of the appointment of independent auditors will be considered by the Audit Committee.
Representatives of PwC will be present at the 2024 Annual Meeting to make a statement if they desire to do so. They will also be available to answer appropriate questions from shareholders.
FOR
The Board of Directors recommends that the shareholders vote FOR ratification of the appointment of PricewaterhouseCoopers LLP as the independent registered public accounting firm of the Company for the fiscal year ending December 31,
|
The Audit Committee’s Report for 20222023 follows.
Merck & Co., Inc.2023 2024 Proxy Statement
Proposal Ratification of Appointment of Independent Registered Public Accounting Firm for | ÷ ÷ ÷ ÷ | 89 |
Audit Committee’s Report
The Audit Committee is made up entirely of independent Directors. The members of the Audit Committee meet the independence and financial literacy requirements of the NYSE and additional heightened independence criteria applicable to members of the Audit Committee under the SEC and NYSE rules. The Audit Committee has adopted, and annually reviews, a charter outlining the practices it follows. The charter complies with all current regulatory requirements.
During 2022,2023, at each of its regularly scheduled meetings (which include meetings scheduled in conjunction with the regular Board meetings, as well as meetings to review the quarterly and annual financial statements filed with the SEC), the Audit Committee met as a group with senior members of the Company’s financial management, the independent auditors and internal auditors. In addition, at each meeting in connection with regular Board meetings, the Audit Committee held separate private sessions with senior management, the independent auditors, internal audit, and the Senior Vice President, Chief Ethics and Compliance Officer to confirm that all were carrying out their respective responsibilities.
The Audit Committee has reviewed and discussed the annual audited financial statements with management. The Audit Committee also has received from the independent auditors the written disclosures and a letter required by the applicable requirements of the Public Company Accounting Oversight Board regarding the independent auditors’ communications with the Audit Committee concerning independence, and has discussed with the independent auditors their independence. Both the independent
auditors and the internal auditors had full access to the Audit Committee.
The Audit Committee met with the independent auditors to discuss their fees, as well as the scope and results of their audit work, including the adequacy of internal controls and the quality of financial reporting. The Audit Committee also discussed with the independent auditors their judgments regarding the quality and acceptability of the Company’s accounting principles, the clarity of its disclosures, and whether its accounting principles and underlying estimates are appropriate, as well as other matters that are required to be discussed by applicable regulatory standards. The Audit Committee reviewed and discussed the audited financial statements with management. Based on the review and discussion referred to above, the Audit Committee recommended to the Board of Directors that the audited financial statements be included in the Company’s Annual Report on Form 10-K filing with the SEC.2023 10-K. Additional information about the Audit Committee and its responsibilities may be found on page 15 of this proxy statement. The Audit Committee Charter is available on our website at www.merck.com/company-overview/leadership/board-of-directors/.
Audit Committee
Douglas M. Baker, Jr.
Pamela J. Craig (Chair)
Stephen L. Mayo, Ph.D.
Paul B. Rothman, M.D.
Christine E. Seidman, M.D.
Kathy J. Warden
Pre-Approval Policy for Services of Independent Registered Public Accounting Firm
As part of its duties, the Audit Committee is required to specifically pre-approve audit and non-audit services performed by the independent auditors to see that the provision of such services does not impair the auditors’ independence. On an annual basis, the Audit Committee also will review and provide pre-approval for certain types of services that may be provided by the independent
auditors without obtaining specific pre-approval from the Audit Committee. If a type of service to be provided by the independent auditors has not received pre-approval during this annual process, it will require specific pre-approval by the Audit Committee. The Audit Committee does not delegate to management its responsibilities to pre-approve services performed by the independent auditors.
Merck & Co., Inc. 20232024 Proxy Statement
| 90 | ç ç ç ç |
Proposal Ratification of Appointment of Independent Registered Public Accounting Firm for |
Fees for Services Provided by the Independent Registered Public Accounting Firm
Fees for PwC, our independent auditors, for 20222023 and 20212022 are as follows:
Type of Payment or Benefit | 2022 ($ in millions) | 2021 ($ in millions) | 2023 ($ in millions) | 2022 ($ in millions) | ||||
Audit Fees(1) | $30.6 | $35.2 | ||||||
Audit Fees(1) | $32.9 | $30.6 | ||||||
Audit-Related Fees(2) | ||||||||
Audit-Related Fees(2) | 5.2 | 7.5 | 3.3 | 5.2 | ||||
Tax Fees(3) | 2.9 | 3.3 | ||||||
Tax Fees(3) | 2.5 | 2.9 | ||||||
All Other Fees(4) | ||||||||
All Other Fees(4) | 0.1 | 0.0 | 0.1 | 0.1 | ||||
Total Fees | $38.8 | $46.0 | ||||||
Total Fees | $38.8 | $38.8 | ||||||
| (1) | Audit Fees included fees for the audit of annual financial statements, the audits of effectiveness of internal control over financial reporting, reviews of quarterly financial statements filed in the reports on Form 10-Q, and statutory audits. |
| (2) | Fees for audit-related services primarily related to employee benefit plan audits, other audit-related reviews, agreed-upon procedures and systems pre-implementation review procedures. |
| (3) | Fees for tax services reported above included approximately |
| (4) | Consisted of fees not included in the Audit, Audit-Related or Tax categories, including fees for reviews performed to maintain compliance with various government regulations relating to the healthcare industry. |
None of the services provided by PwC for fiscal years 20222023 or 20212022 were approved by the Audit Committee pursuant to the waiver of pre-approval provisions set forth in the applicable SEC rules.
Merck & Co., Inc. 20232024 Proxy Statement
| | | | | |
91 |
The Board of Directors Recommends that the Company’s Shareholders Vote AGAINSTProposals 4, 5 and 6.
The text, including any image(s), of the shareholder proposals and supporting statements below appear exactly as received by the Company. All statements and images contained in the proposals and supporting statements are the sole responsibility of the proponent(s) and may contain assertions about the Company or other matters that the Company believes are incorrect, but the Company has not attempted to refute all such assertions. The Board recommends a vote against the shareholder proposals based on the reasons set forth in the Company’s statements in opposition following each of the shareholder proposals.
The addresses of the proponents will be provided promptly upon request. Requests should be sent in writing to the Office of the Secretary, Merck & Co., Inc., 126 East Lincoln Avenue, Rahway, N.J. 07065 U.S.A.
Proposal 4 – Shareholder Right to Act by Written Consent
Kenneth Steiner, of Great Neck, NY, owner of at least $2,000 in market value of the Company’s common stock, has given notice that he intends to present for action at the Annual Meeting the following proposal:
Proposal 4 — Shareholder Right to Act by Written Consent

Shareholders request that our board of directors take such steps as may be necessary to permit written consent by the shareholders entitled to cast the minimum number of votes that would be necessary to authorize an action at a meeting at which all shareholders entitled to vote thereon were present and voting. This includes shareholder ability to initiate any appropriate topic for written consent.
This proposal topic won our 42%-support at the 2020 Merck annual meeting. And the 2020 proposal could have recived a higher vote if it pointed out that a Merck director can only be removed for cause which is another way to say it is impossible for shareholder to remove a director, which makes the right to act by written consent to replace a director all the more valuable to shareholders.
The 42%-support was all the more important because it takes more shareholder conviction to vote for a shareholder proposal, and thereby disregard the Board of Director’s position, than to simply go along with the Board’s position.
Taking action by written consent in place of a meeting is a means shareholders can use to raise important matters outside the normal annual meeting cycle like the election of a new director. For instance 3 Merck directors were each rejected by more than 100 million votes repeatedly since 2020.
The shareholder ability to replace a director by written consent could give Merck directors more of an incentive to improve their performance. Three Merck directors again each received more than 13 7 million against votes in 2023.
Ms. Patricia Russo received 228 million against votes in 2021, 250 million against votes in 2022 and 258 million against votes in 2023. Ms. Russo’s 2023 against votes were up to 50-times the against votes received by a number of other Merck directors. Ms. Russo also repeatedly received the most against votes at General Motors where she is also a director.
Mr. Thomas Glocer, Lead Director, with 137 million against votes violates the most important attribute of a Lead Director—independence. As director tenure goes up director independence goes down. Mr. Glocer has 17-years director tenure at Merck. It is disappointing that Mr. Robert Davis, a relatively new Merck Chairman and CEO, received 157 million against votes.
A shareholder right to act by written consent still affords Merck’s Board of Directors strong protection for a holdout mentality for the status quo during the current rapidly changing business environment. Any action taken by written consent would still need 70% supermajority approval from the shares that normally cast ballots at the Merck annual meeting to equal the required majority vote from all Merck shares outstanding.
Whoever wrote the Board of Director’s text next to the 2020 proposal on this topic apparently thinks there are multiple deep-pocket parties hovering over Merck who can hardly wait to take their chance at getting a 70% approval vote at Merck.
Please vote yes:
Shareholder Right to Act by Written Consent — Proposal 4
Merck & Co., Inc. 2024 Proxy Statement
| 92 | ç ç ç ç | Proposals 4-6 Shareholder Proposals |
Board of Directors’ Statement in Opposition to Proposal 4
The Board has carefully considered the proposal concerning a shareholder right to act by written consent and believes it would not enhance shareholder value and is not in the best interests of the Company and all of its shareholders. The Company routinely monitors and evaluates trends in corporate governance, reviews them against our current practices and structures and regularly asks for and receives input from shareholders and other stakeholders. The Governance Committee considers all this input when reviewing proposals to change our practices. Following a comprehensive review of the Company’s governance structures, the Board recommends voting against the proposal for the reasons discussed below.
Our shareholders can take action in a multitude of ways, including, but not limited to, by calling and acting at a special meeting and by submitting shareholder proposals for consideration at an Annual Meeting of Shareholders.
One way our shareholders can take action is through calling a special meeting. The right to call a special meeting gives shareholders the ability to appropriately act on matters between annual meetings. After substantial outreach to shareholders and consideration of the feedback received as well as the best interests of all shareholders, the Board amended the Company’s By-Laws in 2014 to lower the threshold share ownership required to call a special meeting to 15% (from 25%). Our 15% threshold, to our knowledge, is lower than the thresholds at a majority of S&P 500 companies that afford shareholders the right to call a special meeting. In addition, under New Jersey corporate law, holders of 10% or more of the Company’s stock may submit to the New Jersey Superior Court a request for a special shareholder meeting, which the Court may order upon a showing of good cause. Shareholders also have the right to submit shareholder proposals in accordance with the Company’s By-Laws and Rule 14a-8 under the Securities Exchange Act of 1934, as amended, for consideration at our annual shareholder meetings.
Adopting written consent could disenfranchise many shareholders, is less transparent and less democratic than action at a shareholder meeting, and could create confusion and disruption for shareholders and the Company.
The Company’s Restated Certificate of Incorporation requires that all shareholder action be taken at an annual or special meeting to which all shareholders receive notice and have the ability to express themselves, rather than by written consent, as written consent can potentially exclude many shareholders from the notice and voting process. Our existing requirement ensures that all shareholders have a voice in critical matters affecting the Company, as well as meaningful and structured opportunities to exchange views. If a small subset of shareholders, who have no fiduciary duties to other shareholders and may have short-term or special interests, could act by written consent without a meeting, amendments to the Company’s By-Laws and other significant corporate actions could be taken without all shareholders having an opportunity to provide input on the decision. Actions taken by written consent are less transparent and less democratic than actions taken at a shareholder meeting because shareholder action by written consentcould deprive shareholders of the critical opportunities to receive notice, assess, discuss and vote on the merits of proposed actions, and does not require that a proxy statement containing accurate and complete information be distributed to shareholders before proposed actions. Moreover, allowing shareholders to act by written consent could result in confusion and disruption, as different shareholder groups may solicit multiple written consents simultaneously, some of which may be duplicative or contradictory, which, in turn, could create administrative and financial burdens for the Company without a corresponding benefit to shareholders. In addition, we have received this shareholder proposal in nine of the past thirteen years, and our shareholders have never approved it.
Our Board has demonstrated consistently its commitment to sound corporate governance principles.
The special meeting rights described above are just some of the examples of our Board’s commitment to sound corporate governance principles. Others include:
| • | our By-Laws provide a “proxy access” right that allows a group of as many as 20 shareholders, who have held at least 3% of the outstanding shares for at least 3 years, to nominate individuals representing up to 20% of the Board; |
we have a robust shareholder engagement program through which the Board has received and responded to shareholder feedback;
we do not have a shareholder rights plan (also known as a poison pill);
we do not have any supermajority voting provisions;
11 of our 12 Director nominees are independent;
| • | every director stands for re-election every year; |
our directors are elected by majority vote, other than in contested elections;
we have a strong Lead Independent Director; and
the Board is diverse in terms of gender, race, ethnicity, experience and skills.
AGAINST
The
|
Merck & Co., Inc. 2024 Proxy Statement
Proposals 4-6 Shareholder Proposals | ÷ ÷ ÷ ÷ | 93 |
Business Operations in ChinaGovernment Censorship Transparency Report
National Legal and Policy Center, which holds at least $2,000 in market value of the Company’s common stock, has given notice that it intends to present for action at the Annual Meeting the following proposal:
Proposal 5 — Business Operations in ChinaGovernment Censorship Transparency Report
RESOLVED: Shareholders request the Board to provide a report, published on the Company website and updated at reasonable intervals—omitting proprietary information and at reasonable cost—that beginningspecifies the Company’s policy in response to requests, whether from agencies of the United States Government or from state governments, to aid censorship.
This transparency report would be most valuable if it itemizes requests it has received. Helpful information would include the name and title of the public official making the request; the nature and scope of the request; the date of the request; and the Company’s decision about the request.
Supporting Statement
The United States Government colluded with technology, social media and pharmaceutical companies during the COVID pandemic to censor the speech of American citizens for the purported cause to prevent the spread of so-called “disinformation,”1 which violated their First Amendment rights,2 and suppressed the dissemination of real-time evidence and statistics that could have helped avoid other harms.3
In Bantam Books, Inc. vs. Sullivan (1963), and in other cases, the U.S. Supreme Court has ruled that private entities may not suppress speech at the behest of government.
In a July 18, 2023 letter to Merck & Co., Inc. report annually(“Company”) Chairman/CEO Robert Davis, Rep. Jim Jordan, Chairman of the Committee on the Judiciary of the U.S. House of Representatives, wrote:4
According to shareholdersdocuments obtained by the Committee, personnel from Merck were invited in December 2020 to meet with personnel from other pharmaceutical companies, Executive Branch agencies, and Stanford University to discuss “a coalition to respond to COVID-19 vaccine disinformation.” The entanglement of Executive Branch agencies, third-party organizations, and technology companies to moderate speech-related content online raises questions about the nature and extent to which corporate operations depend on,these actions affected the civil liberties of American citizens. Other reporting indicates that the pharmaceutical industry pressured social media platforms to take down posts....
In the letter, Chairman Jordan also asked Mr. Davis to provide the Judiciary Committee copies of all communications and are vulnerabledocuments the Company possesses regarding its participation in efforts to Communist China, which is a serial human rights violator, a geopolitical threat, and an adversarycensor U.S. citizens.
Evidence shows the Company received overtures from government to censor. For example, the United States. The report should exclude confidential business information but provide shareholders with a sensepurpose of the Company’s reliance on activities conducted within,meeting that included Company personnel apparently helped plan the Stanford Internet Observatory’s “Virality Project.” The Project partnered “with several government agencies,” including the Cybersecurity and under controlInfrastructure Security Agency, the Office of the Communist Chinese government.
Supporting Statement
CNN reportedSurgeon General, and the Centers for Disease Control.15 in 2021The Project “worked directly with employees at Facebook, Google, YouTube, TikTok, and more.... Those companies regularly assured the Project that “Beijing has madethey were addressing the content it clear that multinational corporations have to follow its rules if they wish to operate in the country, and gaining favor can require ... abiding by restrictive regulations ... Many companies have traditionally been willing to play along, given how enticing the giant economy is as a market.flagged.”
Merck & Co., Inc. does business in -and relies on supplies, research and development, labor and services from -entities in Communist China.2
China is an established serial violator of human and political rights.
China is also a hostile adversary of the U.S. for many reasons, including:
China intends to displace the U.S. as the lone global superpower by 2049;
The U.S.Company has committed to defend Taiwan, which China has militaristically asserted is part of its country and may attempt to seize by force;
U.S.-China relations are tense over a number of issues including China’s military expansion; egregious human rights violations; actions related to the COVID pandemic; intellectual property theft; relentless espionage; elimination of freedom in Hong Kong; and environmental pollution.
China has also indicatedadmitted that it would use its industrial capabilities for strategic purposes against adversaries.asks social media companies to censor.6
Communist China, and by extension the companies it controls, were also identified in the U.S. State Department’s 2022 Trafficking in Persons Report as a state sponsor of human trafficking. They are now subject to the Uyghur Forced Labor Prevention Act, which imposes strict verification of parts and products imported from China, that they are not generated from slave labor.
A July 2022 joint statement from the leaders of the British and American domestic intelligence agencies warned that the Communist Chinese Party is the greatest threat to the international order. “We consistently see that it’s the Chinese government that poses the biggest long-term threat to our economic and national security, and by ‘our,’ I mean both of our nations, along with our allies in Europe and elsewhere,” said FBI Director Christopher Wray.
Given the controversial, if not dangerous, nature of doing business in and with China, shareholders have the rightShareholders need to know whether the degreeCompany cooperates with government officials engaged in unconstitutional censorship, opening the Company to whichliability claims by victims, and whether the Company fails to disclose these potential liabilities as material risks in its resources are at risk due to the extent of Merck’s business operations in China, and its dependence on its relationship with the communist government.public filings.
Business Operations in ChinaGovernment Censorship Transparency Report — Proposal 5
1See Disis, Jill & Wang, Selina. “Doing business in China is difficult. A clash over human rights is making it harder,” CNN Business, April 2, 2021. See https://cnn.it/3ef24EI.
2 See https://www.msdchina.com.cn/about.theintercept.com/2023/01/16/twitter-covid-vaccine-pharma/
2See https://nypost.com/2023/03/10/censorship-industrial-comp I ex-uses-power-to-threaten-democracy/
3Seehttps://justthenews.com/politics-policy/coronavirus/wrongly-censored-scientist-presses-covid-19-truth-commission-expose
4See https://judiciary. house. gov/sites/evo-subsites/repubIicans-judiciary.house.gov/files/evo-media-document/2023-07-jd18-j-to-davis-merck.pdf
5Seehttps://public.substack.com/p/stanford-group-helped-us-government
6Seehttps://www.merck.com/news/merck-asks-social-media-companies-to-do-as-much-as-they-can-to-stop-hate-speech-racism-and-discrimination-merck-to-stop-advertising-on-facebook-and-instagram-assess-responses-from--facebook-and-m on i/
Merck & Co., Inc. 20232024 Proxy Statement
| 94 | ç ç ç ç |
Shareholder Proposals |
The Board has carefully considered this shareholder proposal carefully and recommends a vote AGAINST it. The Board believes that the requested report requiring Merck to disclose the nature and extent to which its corporate operations involve or depend on “Communist China” would not provide additional value toAs further discussed below, the Company’s shareholders becauselong-standing mission of saving and improving lives has served throughout the Company’s existing disclosures in its Annual Report on Form 10-K forhistory, as well as today, to guide the year ended December 31, 2022 (the “2022 10-K”) and its other filings with the Securities and Exchange Commission (“SEC”) available at the SEC’s Internet site (www.sec.gov). As a reporting company for purposes of the Securities Exchange Act of 1934, as amended (the “Exchange Act”),decisions the Company is subject to comprehensivemakes and ongoing-business related reporting requirements that include informing its shareholders about its business operations such as in China to the extent they are material.actions it takes. In addition, overseeing risk is an important component of the Board’s engagement on strategic planning as part of the Company-wide Enterprise Risk Management (“ERM”) process overseen by the Audit Committee and, as detailed on pages 18 to 21 of this proxy statement, the Company has extensive disclosuresalready provides significant disclosure regarding its ongoing efforts to increase transparency and the Board’s approach to overseeing risk. Accordingly, the Board believes that the Company provides sufficient insight into the Company’s approach to handling the types of requests contemplated by this proposal, if any, that may be received by the Company.
For more than 130 years, the Company has brought hope to humanity through the development of important medicines and vaccines and has been, and continues to be, guided by a belief in the importance of doing the right thing.
The Company disclosesis unified around its purpose of using the power of leading-edge science to save and improve lives around the world. The Company aspires to be the premier research-intensive biopharmaceutical company in the world. It is at the forefront of research to deliver innovative health solutions that advance the prevention and treatment of diseases in people and animals.
Guided by its purpose, the Company’s values and standards are fundamental to its success and are at the core of its code of conduct, available at https://www.merck.com/company-overview/culture-and-values/code-of-conduct/. The Company’s four values of patients first, respect for people, ethics and integrity, and innovation and scientific excellence represent the core of the Company’s character. These values guide the decisions the Company makes and the actions it takes.
“Patients first” means that everyone at the Company is accountable for delivering high quality products and services. The Company aspires to improve the health and wellness of people and animals worldwide and to expand access to its medicines and vaccines. The Company’s actions must be measured against the responsibility to those who use or need its products.
“Respect for people” recognizes that the Company’s ability to excel depends on the integrity, knowledge, imagination, skill, diversity, safety and teamwork of its employees. The Company works to create an environment of mutual respect, inclusion and accountability, rewards commitment and performance, and is responsive to the needs of its employees and their families.
“Ethics and integrity” focuses on the Company’s commitment to the highest standards of ethics and integrity and the Company’s responsibility to its stakeholders: employees, patients, customers, distributors and suppliers, shareholders, and the communities it serves worldwide. The Company does not take professional or ethical shortcuts.
“Innovation and scientific excellence” emphasizes the Company’s dedication to the highest standard of innovation and scientific excellence. Its research is guided by a commitment to improving health and quality of life. The Company strives to identify and meet the most critical needs of patients and customers through continuous innovation across all areas of its business.
The Company aspires to be transparent about how it operates to earn the trust of its customers and other stakeholders.
As part of this commitment to increasing transparency, the Company proactively provides nonproprietary information about its business operationsbusiness. The Company does so through a variety of mechanisms including through its financial reporting, its annual Impact Report and participation in China and risks related thereto inother voluntary efforts such as CDP (formerly the 2022 10-K.
A foundational principle of the U.S. securities laws is that public companies have an obligation to publicly discloseCarbon Disclosure Project). Additional information that is material to making informed investment decisions. Under Regulation can be found on our website atS-K https://www.merck.com/company-overview/sustainability/transparency-disclosures/ of the Exchange Act, for example, Merck is required to disclose a broad range of information regarding its operations, including a description of its business, its properties and its material risk factors. The 2022 10-K provides robust disclosure under Part I, Items 1 and 1A regarding its business operations in China and any associated material risks.1.
Overseeing risk is an important component of the Board’s engagement on strategic planning and the Company has extensive disclosures regarding the Board’s approach to overseeing risk.
OverseeingAs discussed in more detail in this Proxy Statement, overseeing risk is an important component of the Board’s engagement on strategic planning. The Board’s approach to overseeing risk management leverages the Board’s leadership structure and ensures the Board oversees risk through both a Company-wide approach and specific areas of competency. Specifically, the Board oversees risk through a Company-wide Enterprise Risk Management (“ERM”) process and functioning of Board Committees. The ERM process is reviewed by the Audit Committee of the Board to ensure it is robust and functioning effectively. The ERM process, among other things, seeks to identify emerging risks in business operations and address them appropriately to limit negative consequences to the Company and the data it maintains. Its goal is to provide an ongoing review, implemented across the Company and aligned to Company values and ethics, to identify and assess risk and to monitor risk and agreed-upon mitigating action. If the ERM process identifies a material risk in business operations, it will be elevated through the CEO and the Executive Team to the full Board for consideration. If a risk in business operations transforms into an incident in business operations, the ERM process ensures that effective response and business continuity plans are in place. Through the ERM process, each Board Committee oversees specific areas of risk relevant to the Committee through direct interactions with the CEO, members of the Company’s Executive Team and the heads of relevant business divisions, compliance and corporate functions. A Committee may address risks directly with management or, where appropriate, may elevate a risk for consideration by the full Board or another Board Committee. For a general overview of the ERM process, see pages 18 to 21 of this proxy statement.
Summary
Given Merck’s existing disclosures and the extensive ERM process currently in place, the Board believes that the additional report requested by the proposal is unnecessary and duplicative and not in the best interests of shareholders.
1 The 2022 10-K is available at https://www.merck.com/investor-relations/financial-information/sec-filings/.
|
Proposal 6 – Shareholder Proposal Regarding Access to COVID-19 Products
Oxfam America, Inc., which holds at least $2,000 in market value of the Company’s common stock, has given notice that it intends to present for action at the Annual Meeting the following proposal:
Proposal 6 — Access to COVID-19 Products
RESOLVED: shareholders of Merck & Co, Inc. (“Merck”) ask the Board of Directors to report to shareholders, at reasonable expense and omitting confidential and proprietary information, on whether and how the direct and indirect receipt of public financial support
Merck & Co., Inc. 2023 Proxy Statement
|
|
for development and manufacture of a therapeutic for COVID-19 is being, or will be, taken into account when making decisions that affect access to such products, such as sharing intellectual property through voluntary licenses or setting prices.
Supporting Statement
Merck’s antiviral medicine, molnupiravir, is approved to treat COVID-19.1 Molnupiravir was developed at Emory University using up to $35 million in US government funding,2 and the government maintains “march-in” rights under the Bayh-Dole Act to grant patent licenses to other producers.3 Emory licensed molnupiravir to Ridgeback in March 2020, and Ridgeback entered into a collaboration with Merck for clinical development and manufacturing.4
Merck promised to make the medicine widely available and states that “global access has been a priority.”5 However, Merck has not disclosed how public support factors into decisions that affect access. Setting inaccessible prices could jeopardize the company’s reputation, invite increased regulation and oversight, and ultimately harm investor returns.
This Proposal addresses this risk by asking Merck to explain whether and how public contributions to its products affect how Merck sets prices or the scope of voluntary licenses.
While Merck has signed bilateral licensing agreements and an agreement with the Medicines Patent Pool, those only cover an estimated half of the world’s population and exclude most upper-middle-income developing countries.6 Merck applies a tiered pricing strategy for countries excluded from the voluntary license, but has not disclosed those prices or how the company determines prices that reflect a country’s “ability to finance health care.”7 Tiered pricing typically results in unaffordable prices, especially for middle-income countries.8
Merck’s domestic pricing strategy fails to reflect public support, and the gap between cost and price exposes it to reputational risk: molnupiravir production costs an estimated $20 per course,9 while the company charges approximately $710 in the US, over 35 times the cost of production. For the 3.1 million doses the US government purchased,10 that represents an estimated markup of over $2.1 billion on a treatment developed with public funding. Meanwhile the government is struggling to fund America’s COVID-19 response;11 disparities in access are expected to worsen as a result.12 Merck does not explain how it addresses the relationship between public investment in a product and its pricing and licensing strategy, even in the context of a pandemic.13 If governments cannot trust Merck to ensure access to this publicly funded treatment, governments may set access policies. Policymakers are already scrutinizing how public funding relates to pricing and access strategies, and public funding is already a factor in how the US government will negotiate drug prices.14
Access to COVID-19 Products — Proposal 6
1Seehttps://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.4
2Seehttps://www.wabe.org/emory-researchers-think-they-have-a-drug-to-fight-the-new-coronavirus/; https://www.washingtonpost.com/business/2020/06/11/coronavirus-drug-ridgeback-biotherapeutics/; https://www.keionline.org/36648
3See https://www.keionline.org/36648
4See https://www.businesswire.com/news/home/20200526005229/en/
5See https://www.merck.com/news/merck-and-ridgeback-statement-on-positive-fda-advisory-committee-vote-for-investigational-oral-antiviral-molnupiravir-for-treatment-of-mild-to-moderate-covid-19-in-high-risk-adults/
6Seehttps://msfaccess.org/license-between-merck-and-medicines-patent-pool-global-production-promising-new-covid-19-drug https://www.keionline.org./36648
7Seehttps://www.merck.com/wp-content/uploads/sites/5/2022/08/MRK-ESG-report-21-22.pdf
8 Seehttps://www.researchgate.net/publication/51712884_A_win-win_solution_A_critical_analysis_of_tiered_pricing_to_improve_access_to_medicines_in_
developing_countries
9 Seehttps://scholar.harvard.edu/melissabarber/publications/estimated-cost-based-generic-prices-molnupiravir-treatment-covid-19
10 See https://www.merck.com/news/merck-and-ridgeback-announce-u-s-government-to-purchase-1-4-million-additional-courses-of-molnupiravir-an-investigational-oral-antiviral-medicine-for-the-treatment-of-mild-to-moderate-covid-19-in-a/
11 Seehttps://www.npr.org/2022/09/02/1120746237/white-house-ukraine-covid-19-monkeypox-supplemental-funding
12 See, e.g.,: https://www.healthcaredive.com/news/Medicaid-lose-coverage-hhs-aspe-public-health-emergency/630266/; https://www.nytimes.com/2022/08/28/health/covid-vaccines-money.html; https://fortune.com/2022/08/30/covid-vaccine-treatment-paxlovid-pay-for-cost-free-insurance/;
13 See https://www.nytimes.com/2021/10/27/health/covid-pill-access-molnupiravir.html
14 See https://oversight.house.gov/sites/democrats.oversight.house.gov/files/DRUG%20PRICING%20REPORT%20WITH%20APPENDIX%20v3.pdf; https://aspe.hhs.gov/sites/default/files/private/aspe-files/263451/2020-drug-pricing-report-congress-final.pdf; https://www.congress.gov/bill/117th-congress/house-bill/5376/text
Board of Directors’ Statement in Opposition to Proposal 6
The Board has carefully considered this shareholder proposal and recommends a vote AGAINST it. The Board believes adopting the shareholder proposal is not in the best interests of the Company or our shareholders because preparing the requested report would be duplicative, not an effective use of Merck’s resources and would not provide shareholders with additional meaningful disclosure. Since the inception of Merck’s collaboration with Ridgeback Biotherapeutics (“Ridgeback”) on LAGEVRIO (molnupiravir), which is approved or authorized in several markets for the treatment of certain adults who have been diagnosed with COVID-19, global access to LAGEVRIO has been a priority for the companies. Merck has been transparent about this commitment to provide timely
Merck & Co., Inc. 2023 Proxy Statement
|
|
access to LAGEVRIO globally through our comprehensive supply and access approach, which includes investing in manufacturing at-risk so that supply would be available upon receipt of regulatory authorizations or approvals.1 As further discussed below, this approach and our transparency has been favorably recognized publicly by shareholders that submitted a similar shareholder proposal in 2021, including by means of a public statement that the Company’s license agreement with the Medicines Patent Pool (“MPP”) was “consistent with [their] proposal request”.2 Indeed, other than Oxfam America, each of the other 15 co-proponents of the 2021 proposal did not resubmit a similar proposal this year or last year. In addition, the funding that Oxfam America cites in its proposal as having been received by Emory University was not a factor in the Company’s global access strategy for LAGEVRIO.
Merck is committed to increasing access globally to LAGEVRIO following regulatory authorizations or approvals and has been transparent regarding our approach.
Merck has a long track record of making our vaccines and medicines accessible and affordable globally. Since the early stages of clinical trials of LAGEVRIO, Merck has been committed to a strategy to increase global access to LAGEVRIO recognizing SARS-CoV-2/COVID-19 as an unrivaled scientific and global health challenge. The $35 million in U.S. government funding cited by Oxfam America in its proposal as having been received by Emory University was not a factor in determining the Company’s global access strategy for LAGEVRIO.
Merck invested at-risk – before we had any data on clinical efficacy – to support manufacturing scale-up so that LAGEVRIO would be available upon receipt of regulatory authorizations or approvals. To-date, patients around the world have received approximately 4 million courses of LAGEVRIO, and Merck has supplied LAGEVRIO to more than 40 markets worldwide. Merck also granted voluntary licenses to generic manufacturers and the MPP to make generic molnupiravir available in more than 100 low- and middle-income countries following appropriate regulatory approvals. Through our voluntary licensing agreements with generic manufacturers, more than 5 million courses of generic molnupiravir have been delivered to 22 markets through December 2022. In addition, two licensees, Hetero Labs, Ltd. and Emcure Pharmaceuticals Ltd, have received WHO pre-qualification, an important step in enabling broader access to generic molnupiravir. To supplement the supply from licensed generic manufacturers and bridge to the availability of WHO prequalified generic supply, Merck entered into an agreement with UNICEF to allocate up to 3 million courses of LAGEVRIO to facilitate timely supply to low- and middle-income countries. Merck has also committed 2 million patient courses of LAGEVRIO, available to the U.S. Agency for International Development (USAID) at Merck’s best access price to increase access in lower-income countries. To-date, Merck has also entered into advance purchase and supply agreements for the supply of LAGEVRIO with governments of more than 40 markets worldwide and is currently in discussions with additional governments and is implementing a tiered-pricing approach based on World Bank country income criteria to reflect countries’ relative ability to finance their health response to the pandemic. In September 2022, Merck announced a cooperation framework agreement with Sinopharm Group Co. Ltd., under which Merck granted distribution and exclusive import rights for LAGEVRIO to Sinopharm following authorization. To date, we have shipped more than one million courses of LAGEVRIO to China, with additional shipments planned.
Merck’s efforts regarding global access to LAGEVRIO have been recognized, including by ICCR members that submitted a similar shareholder proposal in 2021.
Charles Gore, executive director of MPP, called the licensing agreement with MPP (the “MPP Agreement”) a “transparent, public health-driven agreement” and noted that it was “MPP’s first voluntary license for a COVID-19 medical technology, and we hope that [Merck]’s agreement with MPP will be a strong encouragement to others.”3 Upon Merck’s announcement of the MPP Agreement, Emory University President, Gregory L. Fenves, issued a statement providing that “the license for molnupiravir to the [MPP] will support global public health and address unmet medical needs — reflecting Emory’s mission to serve humanity.”4 In addition, following Merck’s announcement of the MPP Agreement, a representative of the Province of Saint Joseph of the Capuchin Order of Milwaukee, WI, the lead proponent of the proposal in 2021, contacted Merck personnel to inform Merck that they would not be re-submitting the shareholder proposal again. Seventh Generation Interfaith, an ICCR member involved with the proposal in 2021, issued a statement following the announcement of the MPP Agreement noting that they “do believe that Merck’s decision to make the agreement with MPP is consistent with our proposal request” and that “Merck’s agreement with the MPP goes a long way toward advancing access globally”.5 ICCR itself issued a release stating that members of ICCR and Merck shareholders were “gratified by [the] news” that Merck had entered into the MPP Agreement and that the ICCR members “welcomed the agreement as a precedent-setting event that will hopefully pressure other pharmaceutical companies with COVID-19 entries . . . to follow suit and enter into negotiations with the MPP”.6
1 See press releases in connection with LAGEVRIO available at https://www.merck.com/media/
2 See https://seventhgenerationinterfaith.org/category/iccr/
3 Seehttps://medicinespatentpool.org/news-publications-post/mpp-msd-new-licence-announcement-molnupiravir
4 See https://news.emory.edu/stories/2021/10/molnupiravir_emory_waive_royalties_covid_19/index.html
5 See https://seventhgenerationinterfaith.org/category/iccr/ (noting that they hope that “other pharmaceutical companies follow Merck’s lead and make these lifesaving medications available broadly through mechanisms like the MPP and to do so in terms that are transparent”).
6 Seehttps://www.iccr.org/shareholders-welcome-mercks-decision-share-ip-covid-19-anti-viral-drug (noting also that “We have witnessed the power of the MPP model in advancing access to life-saving medicines . . . Merck has become a first-mover with molnupiravir for COVID-19 and we will be letting its peers know of our expectation that they will soon be following in Merck’s footsteps.”)
|
Merck & Co., Inc. 2023 Proxy Statement
|
|
Proposal 7 – Shareholder Proposal Regarding Indirect Political Spending
Boston Common Asset Management, which holds at least $2,000 in market value of the Company’s common stock, has given notice that it intends to present for action at the Annual Meeting the following proposal:
Proposal 7 — Indirect Political Spending
RESOLVED: The shareholders of Merck & Co., Inc. (“Merck” or “Company”) ask the Company to adopt a policy requiring that any trade association, social welfare organization, or organization organized and operated primarily to engage in political activities that seeks financial support from Merck agree to report to , at least annually, the organization’s expenditures for political activities, including the amount spent and the recipient, and that each such report be posted on Merck’s website.
For purposes of this proposal, “political activities” are (i) influencing or attempting to influence the selection, nomination, election, or appointment of any individual to a public office; or (ii) supporting a party, committee, association, fund, or other organization organized and operated primarily for the purpose of directly or indirectly accepting contributions or making expenditures to engage in the activities described in (i). This proposal does not encompass lobbying spending.
Supporting Statement
As long-term Merck shareholders we support transparency and accountability in corporate electoral spending, including the indirect political spending that is the subject of this proposal. Misaligned or non-transparent funding creates reputational risk that can harm shareholder value. It can also place a company in legal jeopardy. Unless a company knows which candidates and political causes its funds ultimately support, it cannot assure shareholders, employees, or other stakeholders that its spending aligns with core values, business objectives, and policy positions. Without the information requested by this resolution, none of the board, senior management, or shareowners can assess the risks associated with political spending.
The risks are especially serious when giving to trade associations, Super PACs, 527 committees, and “social welfare” organizations – groups that routinely pass money to or spend on behalf of candidates and political causes that a company might not otherwise wish to support. The Conference Board’s 2021 “Under a Microscope” report details these risks, discusses how to effectively manage them, and recommends the process suggested in this proposal.
Media coverage amplifies the risk a company’s blind spending can pose and contributions to third-party groups can also embroil companies in scandal. Public records show Merck has contributed at least $1.3 million in corporate funds to third-party groups dating to the 2020 election cycle. Beneficiaries of this spending have been tied to attacks on voting rights, which we believe run counter to Merck’s stated values.
It is unclear whether the Company and its board received sufficient information from these groups to assess (a) the potential risks for Merck and stockholders, and (b) whether the groups’ expenditures aligned with Merck’s core values, business objectives, and policy positions.
Mandating reports from third-party groups receiving Merck’s political money would demonstrate the Company’s commitment to robust risk management and responsible civic engagement.
We urge a vote FOR the commonsense risk management measures contained in this proposal.
Indirect Political Spending — Proposal 7
Board of Directors’ Statement in Opposition to Proposal 7
The Board has considered this shareholder proposal carefully and recommends a vote AGAINST it because the Company has significant disclosures already regarding the Company’s participation in the political process, including with respect to trade associations, and this proposal lacks a connection to the Company’s political activities. The information requested by the proposal is information about the annual political expenditures of third-party organizations that “seek” financial support from the Company. The Company may not have any kind of a relationship with such organizations and it is not within the Company’s power or authority to guarantee that any such organizations would comply with a policy or request by the Company for this information.
The Company is committed to participating constructively and responsibly in the political process and has significant disclosures about its participation.
Merck & Co., Inc. 2023 Proxy Statement
|
|
The Company aspires to be open and transparent to earn the trust and confidence of our stakeholders. On our website and elsewhere in this proxy statement, we discuss our commitment to participating constructively and responsibly in the political process and to providing clarifying analysis and information regarding the issues that affect our business and patient care. The Company advocates for public policies that foster research into innovative medicines and improve access to medicines, vaccines and health care. Our participation in the political process is guided by the following principles: improving patient access to healthcare, including access to medicines and vaccines, improving access to animal health products, and encouraging innovation. The Company’s public policy positions are determined by senior management with oversight by the Governance Committee. Our political contributions are made in accordance with all applicable laws and Company policies and procedures and are overseen by senior management. The Governance Committee monitors all such contributions, and the full Board receives a bi-annual report.
In addition, the Company publicly discloses and regularly updates information regarding its public policy positions and advocacy expenditures on our website at www.merck.com/company-overview/responsibility/transparency-disclosures/. This information includes the Company’s contributions, categorized by state, candidate and amount, for our corporate political and Political Action Committee contributions where permitted by law, such as in the U.S., Canada and Australia. These disclosures also include information for the past 5 years and are posted semiannually with respect to contributions in the U.S. and annually with to contributions in Canada and Australia. Our website disclosures also include a list of U.S. industry and trade groups in which we are members where our dues are greater than $25,000 and the portion of our dues that these groups use for advocacy and/or political activities, see www.merck.com/company-overview/esg/transparency-disclosures/.
The Proposal is not connected to the Company’s political activities but instead to third parties with whom the Company may not have a relationship.
The proposal requests that the Company “adopt a policy requiring that any trade association, social welfare organization, or organization organized and operated primarily to engage in political activities that seeks financial support from Merck agree to report to [Merck], at least annually, the organization’s expenditures for political activities,” and that the Company post publicly on its website “each such report” (such policy, the “Requested Policy”). It lacks a connection to political activity by the Company, and instead seeks to use the Company as a means for publishing certain political expenditure disclosure from third-party organizations, with respect to which the proponent would not otherwise have access or recourse. By applying to covered organizations that “seek” financial support from the Company, the Requested Policy would apply even to organizations that seek a relationship with the Company that is unrelated to the Company’s political activity (and where the sought form of financial support is not political in nature). For example, if the Company provides financial support to a community organization covered by the Requested Policy and such support is not for political activities, the Requested Policy would require the Company to condition such support on detailed political expenditure reports by such organization or individual. The foregoing is not only impractical and inappropriate, but also beyond the Company’s power to enforce.
The Company cannot compel third parties that only seek financial support from the Company to provide the Company with potentially confidential and proprietary information related to such third parties’ political expenditures.
Based on its express terms, the Requested Policy requires and depends upon action by independent third parties (i.e., certain organizations agreeing to provide, and actually providing, the Company details pertaining to their political expenditures and consenting to the Company’s public posting of such details), and it is not within the Company’s power or authority to guarantee that “any” such organizations would comply with such a policy or request by the Company. In addition, because the Requested Policy broadly applies to organizations that merely “seek” financial support from the Company (as opposed to those organizations that actually receive financial support), the Company would be required to both request and then compel disclosure from third parties to whom it may choose not to contribute and with whom it may not have any relationship whatsoever, as the Company does not provide financial support to every organization that seeks financial support. The decision to publicly report the information requested by the proposal is a matter under the control of the third-party organizations seeking financial support from the Company, not the Company itself. The Company has no power to direct or mandate that third-party organizations seeking financial support agree, simply as a condition of their request for financial support (which may be completely unsolicited by the Company), to provide annual disclosures to the Company that will subsequently be publicly disclosed by the Company.
|
Proposal 8 – Shareholder Proposal Regarding Patents and Access
The Province of Saint Joseph of the Capuchin Order and other co-filers, each of whom holds at least $2,000 in market value of the Company’s common stock, have given notice that they intend to present for action at the Annual Meeting the following proposal:
Merck & Co., Inc. 2023 Proxy Statement
|
|
Proposal 8 — Patents and Access
RESOLVED: that shareholders of Merck & Co., Inc. (“Merck”) ask the Board of Directors to establish and report on a process by which the impact of extended patent exclusivities on product access would be considered in deciding whether to apply for secondary and tertiary patents. Secondary and tertiary patents are patents applied for after the main active ingredient/molecule patent(s) and which relate to the product. The report on the process should be prepared at reasonable cost, omitting confidential and proprietary information, and published on Merck’s website.
Supporting Statement
Access to medicines, especially costly specialty drugs, is the subject of consistent and widespread public debate in the U.S. A 2021 Rand Corporation analysis concluded that U.S. prices for branded drugs were nearly 3.5 times higher than prices in 32 OECD member countries.1 The Kaiser Family Foundation has “consistently found prescription drug costs to be an important health policy area of public interest and public concern.”2
This high level of concern has driven policy responses. The Inflation Reduction Act empowers the federal government to negotiate some drug prices.3 State measures, including drug price transparency legislation, copay caps, and Medicaid purchasing programs, have also been adopted.4 The House Committee on Oversight and Reform (the “Committee”) launched an investigation into drug pricing in January 2019.5
Intellectual property protections on branded drugs play an important role in maintaining high prices and impeding access. When a drug’s patent protection ends, generic manufacturers can enter the market, reducing prices. But branded drug manufacturers may try to delay competition by extending their exclusivity periods.
Among the abuses described by the Committee’s December 2021 report is construction of a “patent thicket,” which consists of many “secondary patents covering the formulations, dosing, or methods of using, administering, or manufacturing a drug” granted after the drug’s primary patent, covering its main active ingredient or molecule, has been granted.6 In June 2022, citing the impact of patent thickets on drug prices, a bipartisan group of Senators urged the U.S. Patent and Trademark Office to “take regulatory steps to . . . eliminate large collections of patents on a single invention.”
Merck markets cancer drug Keytruda. According to I-MAK, Merck has filed for 95 secondary patents on Keytruda.7 Forty percent of Merck’s patent applications on Keytruda relate to “methods of production and processes that can be used to manufacture the drug,” which can thwart competition even after the primary patent on the drug has expired.8
In our view, a process that considers the impact of extended exclusivity periods on patient access would ensure that Merck considers not only whether it can apply for secondary and tertiary patents but also whether it should do so. Merck’s current approach subjects the company to reputational risks and potential regulatory blowback resulting from high drug prices and perceptions regarding abusive patenting practices.
Patents and Access — Proposal 8
1See www.rand.org/news/press/2021/01/28.html
2Seewww.kff.org/health-costs/poll-finding/public-opinion-on-prescription-drugs-and-their-prices/
3Seewww.kff.org/medicare/issue-brief/explaining-the-prescription-drug-provisions-in-the-inflation-reduction-act/
4Seewww.americanprogress.org/article/state-policies-to-address-prescription-drug-affordability-across-the-supply-chain/
5See oversight.house.gov/sites/democrats.oversight.house.gov/files/DRUG%20PRICING%20REPORT%20WITH%20APPENDIX%20v3.pdf, at i.
6See oversight.house.gov/sites/democrats.oversight.house.gov/files/DRUG%20PRICING%20REPORT%20WITH%20APPENDIX%20v3.pdf, at 79.
7Seehttp://www.i-mak.org/wp-content/uploads/2021/05/i-mak.keytruda.report-2021-05-06F.pdf, at 3.
8Seehttp://www.i-mak.org/wp-content/uploads/2021/05/i-mak.keytruda.report-2021-05-06F.pdf, at 3.
Board of Directors’ Statement in Opposition to Proposal 8
The Board has considered this shareholder proposal carefully and recommends a vote AGAINST it. The Board believes adopting the shareholder proposal is not in the best interests of the Company or our shareholders because the Company has significant disclosures regarding (1) access to its products, including Access to Health Guiding Principles that guide the Company’s global approach to access to health, (2) pricing transparency in the U.S., which is the country on which the shareholder’s supporting statement focuses, and (3) the importance of patent protection to innovation and the Company’s business, including through disclosures in the Annual Report on Form 10-K for the fiscal year ended December 31, 2022 (the “2022 10-K”), and the Company’s process for determining whether to apply for patent protection involves a fact-specific and complicated scientific and legal analysis for every product-related invention that has the potential of being patented.
Access to Health: in collaboration with key stakeholders, Merck works to ensure its science advances health care and that its products are accessible and affordable to those in need.
As discussed on page 20 of this proxy statement and further discussed in our ESG Progress Report, access to health is a key focus areas for Merck. Merck aspires to improve access to health by discovering, developing, and providing innovative products and services
Merck & Co., Inc. 2023 Proxy Statement
|
|
that save and improve lives. We also recognize a robust health care ecosystem expands Merck’s impact, opportunities and value. Our Access to Health Guiding Principles, available at https://www.merck.com/company-overview/esg/esg-resources/, guide our global approach to access to health through four principles: Discovery and Invention, Availability, Affordability, and Strengthening Systems and Addressing Inequity. Merck discovers and invents medicines and vaccines that address vital global health needs where we can have the greatest impact, now and in the future. In 2022 alone, our research and development (R&D) spend was $13.5 billion. Embedded within our research and development process, we systematically evaluate our candidates to identify the potential to address significant public health burden and unmet medical needs in underserved health care settings. This evaluation process informs our product access strategies, with the goal of making our medicines and vaccines available to as many people as possible through sustainable solutions. To facilitate this access, we undertake a systematic evaluation at the onset of Phase 2 clinical studies to determine a candidate’s potential to meet unmet medical needs in low- and middle-income countries. Our approach involves evaluating the level of disease burden that exists, the availability of alternative medications and the appropriateness of our candidates to improve public health. For candidates with significant potential in underserved settings, access planning may start in the pre-clinical phase. Understanding where health system infrastructure and funding mechanisms are in place is an important component of enabling safe and effective usage and facilitating meaningful patient access.
Pricing transparency in the U.S.
We have a long history of making our medicines and vaccines accessible and affordable through responsible pricing practices and industry-leading patient access programs. As part of the Company’s ongoing commitment to transparency about our business operations, and to help people better understand our pricing practices in the U.S., in 2017 we began disclosing information about the price of medicines across our portfolio in the U.S. (See our Pricing Transparency Report available at https://www.merck.com/company-overview/esg/esg-resources/). This information includes changes in average annual list and net prices across our product portfolio since 2010. The disclosure also includes the average discount rate across our portfolio each year. We are working to bring our medicines and vaccines to more people around the world in ways that are as accessible and affordable as possible for the patients who need them. While each individual situation varies based on factual circumstances and market dynamics, generally we consider: (a) value provided to patients; (b) value provided to health care systems; (c) unmet need; (d) access; (e) R&D sustainability; and (f) competition. Additional information about our activities can be found in our ESG Progress Report.
Patents play an important role in innovation and the Company’s business and the Company conducts a fact-specific and complicated scientific and legal analysis for every product-related invention that has the potential of being patented.
Developing a new medicine begins with basic and other preclinical research, long before any patent applications are filed. The risks of biopharmaceutical R&D are significant with most investigational biopharmaceuticals failing to obtain FDA approval1, and fewer than 12% of drugs that enter Phase I clinical trials are approved by FDA and marketed.2 The 2022 10-K describes the risks facing the Company in this area (see page 34 of the 2022 10-K). While a patent directed to the active ingredient of a drug product is typically filed first, there is often continued investment in R&D, which may occur before or after the drug product is approved, to explore additional potential benefits for patients. In some cases, additional research determines that a medicine can also be used to treat different states of the same disease, such as earlier stages of cancer. Additional research may also demonstrate the medicine can be used to treat completely different conditions including different forms of cancer, or different diseases altogether. In other cases, additional research may lead to increased safety or effectiveness, new dosage forms, or new forms of administration of a medicine that can improve patient adherence or convenience, leading to better patient outcomes. Patents play a key role in safeguarding the investments made to research and develop additional uses, which may require a significant investment and take three to twelve years to develop.3
The 2022 10-K contains significant disclosures regarding patent protection and its importance to the Company’s business (see pages 11-13 of the 2022 10-K). The Company conducts a fact-specific and complicated scientific and legal analysis for every product-related invention that has the potential of being patented as part of its process for determining whether to apply for patent protection. The analysis is performed by the Company’s experienced patent attorneys (each of whom holds a science degree, or otherwise has an extensive scientific background, in addition to a law degree) in close collaboration with science and medical professionals. The Company evaluates its innovations for potential patenting using similar processes deployed by patent offices when examining the merits of applications for patentability, including performing a complicated evaluation of the innovation for newness (novelty) and non-obviousness relative to what is publicly known. Although newness or novelty is often a relatively straightforward concept, non-obviousness (called “inventive step” outside the U.S.) is a highly fact-specific inquiry, from the perspective of a person skilled in the subject matter, of whether the invention is sufficiently different from prior public disclosures, and may include evaluating a host of what are called “secondary” considerations that may bolster a finding of non-obviousness (e.g., the failure of others to achieve the same result, or that the invention meets a long felt but unresolved need). The Company undertakes that analysis for each patent application it considers filing in addition to applying the Company’s framework intended to affirm the biopharmaceutical industry’s commitment to innovation and keep patients at the heart of our efforts.
1See PhRMA, The Dynamic U.S. Research and Development Ecosystem, at 2 (2021).
2See Research and Development in the Pharmaceutical Industry, Congressional Budget Office, at 16-17 (Apr. 2021).
3See PhRMA, Comments of the Pharmaceutical Research and Manufacturers of America in Response to the USPTO’s Request for Comments on USPTO Initiatives to Ensure the Robustness and Reliability of Patent Rights, at 3 (2023) citing Erika Lietzan, Paper Promises For Drug Innovation, 26 GEO. MASON L. REV. 168, 177-78 (2018); Benjamin N. Roin, Solving the Problem of New Uses, at 5 (Oct. 14, 2016).
|
Merck & Co., Inc. 2023 Proxy Statement
|
|
Proposal 9 – Shareholder Proposal Regarding a Congruency Report of Partnerships with Globalist Organizations
The National Center for Public Policy Research, which holds at least $2,000 in market value of the Company’s common stock, has given notice that it intends to present for action at the Annual Meeting the following proposal:
Proposal 9 — Congruency Report of Partnerships with Globalist Organizations
RESOLVED: We request that Merck & Co., Inc. (the “Company”) publish a report, at reasonable expense, analyzing the congruency of voluntary partnerships with organizations that facilitate collaboration between businesses, governments and NGOs for social and political ends against the Company’s fiduciary duty to shareholders.
Supporting Statement
Merck does not list the World Economic Forum (WEF), Council on Foreign Relations (CFR) or Business Roundtable (BR) among its partners or as recipients of contributions;1 however, WEF and CFR do list the Company as a partner2 and BR lists CEO Robert Davis as a member.3 Why the inconsistency? Why is the Board concealing these partnerships, amongst others, from shareholders?
The Company’s legal duty as a New Jersey For-Profit Corporation requires the Company to serve the interests of its shareholders. Because the Company is not a B-Corporation,4 all additional Company actions and expenditures with third parties (while permissible) must be shown by the Board to be congruent with the interests of shareholders and the Company’s fundamental purpose of making and selling healthcare products.
However, the agendas of WEF, CFR and BR are antithetical with the Company’s fiduciary duty. This obliges the board to explain how partnerships with such organizations serve the interests of shareholders (rather than Directors).
WEF describes itself as an “international organization for public-private cooperation,” and that it was “founded on the stakeholder theory, which asserts that an organization is accountable to all parts of society.”5
Similarly, CFR describes itself as a “membership organization” for both “government officials” and “business executives” on an international scale.6 And BR pretended to redefine “the purpose of a corporation” such that a corporation ought to cater to the special interests of selected “stakeholders” rather than the fundamental interests of its owners, the shareholders.7
Those agendas are incongruent with the interests of shareholders and the traditional—and legally binding—definition of a corporation. The more the Board pays favor to hand-picked “stakeholders,” the less it’s accountable to capital-providing shareholders. In partnering with WEF, CFR and BR, then, shareholders are funding the movement designed to debase their own influence within the Company.
But most importantly, it’s the radical agendas of these organizations that makes our partnerships with them so troubling, not to mention inconsistent with the values of most Merck shareholders.
For example, WEF openly advocates for transhumanism,8 abolishing private property,9 eating bugs,10 social credit systems,11 “The Great Reset,”12 and host of other blatantly Orwellian objectives.
Most shareholders are unaware (since the Board hides it from them) that their capital is in part being used to pursue this anti-human, anti-freedom agenda. Moreover, none of this is congruent with the Company’s basic purpose of providing value to shareholders by making and selling healthcare products.
Congruency Report of Partnerships with Globalist Organizations — Proposal 9
1See https://www.merck.com/company-overview/esg/philanthropy/; https://www.merck.com/wp-content/uploads/sites/5/2022/08/MRK-ESG-report-21-22.pdf;
2See https://www.weforum.org/partners; https://www.cfr.org/membership/corporate-members
3Seehttps://www.businessroundtable.org/about-us/members
4Seehttps://law.justia.com/codes/new-jersey/2018/title-l4a/chapter-18/section-14a-18-5/
5See https://www.weforum.org/about/world-econornic-forum/
6See https://www.cfr.org/about
7See https://www.businessroundtable.org/purposeanniversary
8See https ://www.weforum.org/about/the-fourth-industrial-revolution-by-klaus-schwab
9See https://web.archive.org/web/20200919112906/https://twitter.com/wef/status/799632174043561984
10See https ://www.weforum.org/agenda/2021 /07 /why-we-need-to-give-insects-the-role-they-deserve-in-our-food -systems/
11Seehttps://www.weforum.org/reports/identity-in-a-digital-world-a-new-chapter-in-the-social-contract
12Seehttps://www.weforum.org/focus/the-great-reset
Merck & Co., Inc. 2023 Proxy Statement
|
|
Board of Directors’ Statement in Opposition to Proposal 9
The Board has considered this shareholder proposal carefully and recommends a vote AGAINST it. The Board believes that the requested report “analyzing the congruency of voluntary partnerships with organizations that facilitate collaboration between businesses, governments and NGOs for social and political ends against the Company’s fiduciary duty to shareholders” would be costly and time-consuming for the Company to prepare and would not provide additional value to the Company’s shareholders. The proposal does not define what is meant by “organizations that facilitate collaboration between businesses, governments and NGOs for social and political ends”, but the proposal’s supporting statement references three organizations, the Council on Foreign Relations (“CFR”), the World Economic Forum (“WEF”), and the Business Roundtable (“BRT”). The Company is a member of both the CFR and the WEF and our Chairman and CEO, Robert M. Davis, is a member of the BRT.
As a company, Merck runs to, not from, the world’s biggest health challenges.
From finding solutions for some of the world’s most debilitating diseases, to getting the Company’s medicines and vaccines to those in need and building more effective health systems, the Company’s long-standing mission of saving and improving lives has served throughout Merck’s long history, as well as today, as a foundation for driving long-term value for the Company and our shareholders. Along with investment and endless invention, partnerships play a key role in helping us ensure our products are accessible and affordable to those in need and overcome barriers to providing a healthier future for all. In addition, the Company already provides significant disclosures regarding how the Board exercises its responsibility to oversee the Company, including with respect to the Company’s strategy, which, in turn allows shareholders to review how the Board is exercising its legal obligations.
The Company’s long-standing mission of saving and improving lives has throughout its history served and continues to serve as the foundation for the Company’s overall strategy.
For more than 130 years, Merck has been driven by its mission of saving and improving lives around the world and its purpose of using the power of leading-edge science to achieve that mission. This purpose has served, and continues to serve, as the foundation for the Company’s overall strategic framework. Indeed, as George W. Merck stated in 1950 during a speech at the Medical College of Virginia at Richmond, “We try to remember that medicine is for the patient. We try never to forget that medicine is for the people. It is not for the profits. The profits follow, and if we have remembered that, they have never failed to appear.”
Throughout its history, Merck has brought hope to humanity through the development of important medicines and vaccines. The Company aspires to be the premier research-intensive biopharmaceutical company in the world—and today, the Company is at the forefront of research to deliver innovative health solutions that advance the prevention and treatment of diseases in people and animals.
Partnerships play a role in overcoming barriers to providing a healthier future for all.
Barriers to access and quality care exist in many parts of the world. The Company has a role to play in helping to ensure our products are accessible and affordable to those in need and partnerships are important in this regard. For example, the Company is steadfast and dedicated to discovering, developing, supplying and delivering vaccines to help prevent diseases around the world. However, to achieve the broadest possible access and distribution to its vaccines, the Company cannot do it alone and works with governments, international health and development organizations, donor groups, nongovernmental organizations, and others to create new ways to improve vaccine access. In addition, because every community is different, the Company commits to working with organizations that are a part of those communities to help make certain diseases a thing of the past.
The Company already provides significant disclosure about the how the Board effectively exercises its responsibility to oversee the Company.
As discussed on page 11 of this proxy statement, the Board believes that good corporate governance is essential to achieving long-term shareholder value and is committed to governance policies and practices that serve the interests of our Company and its many stakeholders. For this reason, we devote considerable time and resources to making sure that our policies reflect our values and business goals, we have an effective corporate governance structure, and we operate in an open, honest and transparent way. In addition, we evaluate our practices against prevailing best practices as well as emerging and evolving topics identified in a variety of ways, including through shareholder engagement and corporate governance organizations. The Board is also fully engaged and involved in the Company’s strategic planning process. By exercising sound and independent business judgment on the strategic issues that are important to the Company’s business, the Board facilitates Merck’s long-term success. Overseeing risk is an important component of the Board’s engagement on strategic planning. The Board’s approach to overseeing risk management leverages the Board’s leadership structure and ensures the Board oversees risk through both a Company-wide approach and specific areas of competency. A summary of this risk oversight approach is included on page 18 of this proxy statement.
AGAINST
The Board of Directors recommends that the shareholders vote AGAINST this proposal.
|
Merck & Co., Inc. 20232024 Proxy Statement
Shareholder Proposals | ÷ ÷ ÷ ÷ | 95 |
106 – Shareholder Proposal Regarding an Independent Board Chairmana Report on Respecting Workforce Civil Liberties
Kenneth Steiner, of Great Neck, NY, owner ofThe Bahnsen Family Trust dated July 14, 2003, which holds at least $2,000 in market value of the Company’s common stock, has given notice that heit intends to present for action at the Annual Meeting the following proposal:
Proposal 106 — Independent Board ChairmanReport on Respecting Workforce Civil Liberties
RESOLVED: Shareholders request that the Board of Directors adoptconduct an enduring policy,evaluation and amendissue a civil rights and non-discrimination report within the governing documents as necessary in ordernext year, at reasonable cost and excluding proprietary information and disclosure of anything that 2 separate people holdwould constitute an admission of pending litigation, evaluating how Merck’s policies and practices impact employees and prospective employees based on their religion (including religious views) or political views, and the officerisks those impacts present to Merck’s business.
Supporting Statement
Merck and Co. is one of the Chairman and the office of the CEO.
Whenever possible, the Chairman of the Board shall be an Independent Director.
The Board has the discretion to select a Temporary Chairman of the Board who is not an Independent Director to serve while the Board is seeking an Independent Chairman of the Board on an accelerated basis.
It is a best practice to adopt this policy soon. However this policy could be phased in when there is a contract renewal for our current CEO or for the next CEO transition.
The roles of Chairman and CEO are fundamentally different and should be held by 2 directors, a CEO and a Chairman who is completely independent of the CEO and Merck. The job of the CEO is to manage the company. The job of the Chairman is to oversee the CEO and management.
A Lead Director is no substitute for an independent Board Chairman. A lead director is not responsible for the strategic direction of the company. And a Chairman/CEO can ignore the advice and feedback from a lead director.
Plus there are weaknesseslargest companies in the roleUnited States and employs over 69,000 people. As a major employer, Merck should respect the free speech and religious freedom of its employees. Merck lead director:
Can only approve meeting agendas and the information sentis legally required to the Board, but apparently no role in the development of meeting agendas and information.
Can only approve meeting schedules, but apparently no role in the development of meeting schedules, plus the scope of the approval is only to make sure there is enough time.
At its 2020 annual meeting Lowe’s (LOW) directors said that having a separate Chairman and Chief Executive Officer affords allows the Chairman to devote his time and attention to Board oversight.
The Merck board needs attention. Ms. Patricia Russo received 228 millioncomply with many laws prohibiting discrimination against votes in 2021 and 250 million against votes in 2022. These 2 against votes were up to 38-times the against votes received by other Merck directors. Ms. Russo was also the leader in against votes at Merck in 2020. Ms. Russo also received the most against votes at General Motors where she is also a director.
Mr. Thomas Glocer, Lead Director, violates the most important attribute of a Lead Director -independence. As director tenure goes up director independence goes down. Mr. Glocer has 16-years director tenure at Merck. 16-years director tenure means that the skills Mr. Glocer had 16-years ago and his intervening skills may no longer be relevant to Merck.
Plus management fails to give shareholders enough information on this topic to make a more informed decision. There is no management comparison of the exclusive powers of the Office of the Chairman and the de minimis exclusive powers of the Lead Director.
Please vote yes:
Independent Board Chairman — Proposal 10
Board of Directors’ Statement in Opposition to Proposal 10
The Board has carefully considered the shareholder proposal and, for the reasons described below, believes adopting it is not in the best interests of Merck’s shareholders. The Board’s current leadership model provides strong, consistent and experienced leadership, as well as robust, effective and independent Board oversight of management, and allows the Board appropriate flexibility to determine the best leadership structure based on facts and circumstances at a given time.
Providing our independent and skilled Board the flexibility to determine our leadership structure at a given time and based on relevant circumstances best serves our shareholders and our Company.
Currently, the Board is led by Robert M. Davis, who serves as the Chairman of the Board and CEO of Merck, and by Thomas H. Glocer, an independent Director, who serves as the Board’s Lead Director. Our Board believes that our shareholders and our Company are best served by allowing the Board to exercise its judgment regarding the most appropriate leadership structure of the Company and the Board at a given time. The most effective leadership structure at a given time will dependemployees on a variety of factors, including butreligion and sometimes political affiliation.
Respecting diverse views also allows Merck to attract the most qualified talent, promote a healthy and innovative business culture, serve its diverse customer base, and contribute to a healthy economic market and marketplace of ideas.
Despite this, the 1792 Exchange’s 2023 report1 notes that Merck does not limitedprovide its employees with protection against viewpoint discrimination. While the Company expressly condemns2 and prohibits discrimination based on a variety of characteristics, including “skin colour, race, nationality, disabilities, religion, sexual orientation” and others, it maintains no such protection against employees of diverse political beliefs.
Many companies also alienate their own employees by taking divisive stances on political issues. For example, many companies have adopted radical stances and policies on abortion and sex reassignment surgery, termed ‘gender-affirming care.’ Merck is one such example. The Company indirectly funds abortion provider Planned Parenthood3 and has pledged4 coverage for “medically necessary transition-related care” for its employees and their children. The 2023 Viewpoint Diversity Index5 also found that 78% of scored companies discriminate against religious nonprofits in their charitable giving and 63% give money to legislation that undermines fundamental First Amendment freedoms. According to the leadership, skillsFreedom at Work survey, 60% of employees were concerned that their company would punish them for expressing their religious or political views at work, and experience54% said they feared the same for sharing these views even on their private social media accounts.6 Such concerns become relevant in the case of eachMerck. As per the 1792 Exchange’s 2023 report,7 the Company’s gift matching policy8 expressly excludes religious groups.
Companies may also face additional legal liability for DE&I programs that make distinctions based on race or that discriminate based on religious practices, per the recent Supreme Court decisions in Students for Fair Admission v. Harvard and Groff v DeJoy. In light of these risks, the CEO, the independent Lead DirectorCompany must take immediate steps to assess potential shortcomings and the other members of the Board, as well as the needs of the business. Basedact to remedy these concerns.
Report on these factors, the Board is best positioned to identify the individual who has the skills and commitment necessary to perform the role of Chairman most effectively at the time.Respecting Workforce Civil Liberties — Proposal 6
1Seehttps://1792exchange.com/company/merck-co/
2Seehttps://www.merck.com/wp-content/uploads/sites/5/2020/04/Policy_2019_Human-Rights_MERCK.pdf
3Seehttps://www.merck.com/wp-content/uploads/sites/5/2021/07/MSD_FINAL-Charitable-2017-Full_Year_Transparency_Report.pdf
4Seehttps://www.hrc.org/resources/buyers-guide/merck-2
5See https://www.viewpointdiversityscore.org/
6See https://www.viewpointdiversityscore.org/polling
7Seehttps://1792exchange.com/company/merck-co/
8Seehttps://www.merck.com/wp-content/uploads/sites/5/2020/10/Grant-Application-Guidelines.pdf
Merck & Co., Inc. 20232024 Proxy Statement
| 96 | ç ç ç ç |
Shareholder Proposals |
The independent members of the Board review the Board’s leadership structure at least annually. In 2021, the Board unanimously elected Robert M. Davis to succeed Kenneth C. Frazier as CEO. At that time, the Board determined that it was in the Company’s best interest for the roles of CEOhas carefully considered this shareholder proposal and Chairman to be held by separate individuals, and for Mr. Frazier to continue as Executive Chairman. From then until Mr. Frazier’s retirement as Executive Chairman at the end of 2022, the independent Directors gave significant consideration to the Board’s leadership structure and also had the opportunity to work directly with Mr. Davis. During this period, the Board found Mr. Davis to be an innovative leader with deep understanding of the Company and its industry and well-suited to serve as Chairman of the Board. Accordingly, effective December 1, 2022, Mr. Davis was unanimously elected by the Board to serve as the Chairman, resulting in the following leadership structure for the Company: Mr. Davis serving as Chairman, CEO and President and Mr. Tom Glocer, an independent director appointed by the independent members of the Board serving as Lead Director of the Board. Each of the Chairman and independent Lead Director role has clearly delineated responsibilities: as Chairman, Mr. Davis presides over meetings of the Board and shareholders, focuses on Board operations, and is in charge of the general supervision, direction and strategy of the business and affairs of the Company subject to the Board’s extensive oversight; and Mr. Glocer, as independent Lead Director, hasrecommends a clear mandate and significant authority as set forth in the Polices of the Board and highlighted below.vote AGAINST it. The Board believes that having Mr. Davis serve as Chairmanthe report requested by this proposal would be costly and CEO adds strategictime-consuming for the Company to prepare and operational perspectivewould not provide additional value to the Chairman role because he can draw on detailed institutional knowledgeCompany’s shareholders. As further discussed below, the Company’s policies, practices, and procedures demonstrate that diverse viewpoints are respected and encouraged and are an essential part of advancing our business. In light of our demonstrated commitment to developing and maintaining a diverse and inclusive workforce, the Board believes that adopting this shareholder proposal is unnecessary and not in the best interests of the Company or our shareholders.
The Company supports transparency and industry experience, while at the same time having strong independent oversight with Mr. Glocer as independent Lead Director vested with key dutiesaccountability in fostering a culture that is inclusive and responsibilities and four independent Board committees chaired by independent Directors.supportive of its employees.
Our existing governance practices and current leadership structure promote effective and independent Board oversight.
Our strong corporate governanceThe Company already provides extensive disclosures regarding its policies and practices, includingstrategy to foster a culture that is inclusive and supportive of all employees and to enhance the substantial percentagediversity of independent Directors on our Board,its workforce. These disclosures include the information discussed herein as well as additional information that can be found in our most recent Impact Report (available at https://www.merck.com/company-overview/sustainability/) and on our website, including at https://www.merck.com/company-overview/diversity-and-inclusion/.
From an accountability perspective, in addition to financial and pipeline metrics, the robust dutiesCompany’s 2023 Scorecard includes metrics regarding engagement and inclusion of the Company’s employees and enabling access to the Company’s innovative portfolio to patients around the world. As more fully described elsewhere in this Proxy Statement, the Company’s Scorecard helps translate the Company’s strategic priorities into operational terms that enable tracking and measurement of our independent Lead Director, empower our independent Directorsprogress and performance against annual operating goals and the long-term strategic drivers of sustainable value creation. The addition of a metric tied to effectively exercise Board oversight. As further detailedinclusion of the Company’s employees demonstrates the Board’s commitment to accountability in this area.
The Company’s longstanding commitment to diversity, equity and inclusion is based on its belief that unique, passionate perspectives are critical to innovation and, in turn, provide a competitive advantage for the Company.
Enhancing diversity in the Board Leadership Structure sectionCompany’s employee population betters the Company’s understanding of this proxy statement,its customers, promotes the significant authorityinclusion of diverse populations in its clinical trials, and responsibilitiesencourages the innovation that drives the Company’s business. Put simply, diversity and inclusion among the Company’s workforce is a business imperative for the Company, and the Company’s success is built on a culture that embraces employees’ different perspectives and values their contributions. Fostering a culture that is inclusive and supportive is therefore fundamental to the Company’s business. As part of its efforts to listen to employees and to maintain a satisfying and productive work environment, the independent Lead DirectorCompany routinely surveys all employees to learn about their perspectives on the business and on how the Company is responding to the needs of our global workforce.
The Company’s Employee Pulse Surveys are clearly defineda key employee feedback mechanism. Conducted multiple times a year, these all-employee engagement surveys allow the Company to measure employees’ perceptions on inclusion and include, butengagement as well as other critical workforce issues.
Embodying our commitment to different constituencies and enhancing communication and belonging, the Company also supports 10 Employee Business Resource Groups that foster retention, facilitate growth, provide mentorship and strengthen networks while providing culturally relevant insights and sensitivities that help drive our success.
“Ethics and integrity” is one of four key values that unite the Company’s workforce and represent who we are not limited to:as a Company.
The Company’s workforce is united by four key values that represent who we are as a Company and how the Company’s employees work together in unison:
The authority to call meetings of the independent Directors;Patients first
Presiding at all meetings of the Board at which the Chairman is not present, including executive sessions of the independent Directors;Respect for people
Serving as the principal liaison on board-wide issues between the independent DirectorsEthics and the Chairman/CEO;integrity
Approving meeting agendasInnovation and the information sentscientific excellence
As a company, Merck is fully committed to operating responsibly to enable a safe, sustainable and healthy future for people and communities everywhere. The Company’s code of conduct setting forth its value and standards is available at https://www.merck.com/company-overview/culture-and-values/code-of-conduct/.
The Company communicates regularly with its employees to encourage a speak-up culture and to ensure they understand how to report potential concerns. The Company has a goal of fostering a “Speak Up” culture by maintaining or exceeding its current percentage of employees responding favorably to the Board, including supporting material for meetings;
Approving meeting schedules“Willingness to ensure there is sufficient time for discussion of all agenda items;
Being available for consultation and direct communication with major shareholders, as appropriate;
Serving as a liaison between the Board and shareholders on investor matters;
Leading the annual performance evaluations of the Board and the Chairman/CEO; and
Leading the Chairman and CEO succession planning process.
Moreover, all four standing Board committees are composed solely of independent Directors and are led by independent chairs. These four independent Board committees are responsible for the oversight of many critical matters, such as evaluating the Chairman/CEO’s performance, overseeing the integrity ofreport” question in the Company’s financial statements, monitoring our risk-management program, designing our executive compensation program, developing the strategiesquarterly Employee Pulse survey as an annual average and operations forreported progress against this goal in its most recent Impact Report. The Company has also developed an Ethics and Integrity Culture-Building Assessment and Listening Program that aims to build and sustain trust between employees and management and enhance the Company’s research and development of pharmaceutical products and vaccines, reviewing policies and practices of the corporate political contributions program, and nominating new directors. As a non-independent Director, Mr. Davis is not a member of any Board committee.
Summary
In the judgment of the Board, its current leadership structure strikes a balance between strong and consistent executive leadership and effective independent oversight that is right for Merck at this time. The proposal seeks to eliminate the Board’s ability to exercise its judgment regarding the Board leadership arrangements that would best serve the interests of our Company and our shareholders at any particular time and replace it with an inflexible approach to Board leadership that would apply at all times whether or not it is then appropriate for our Company and our shareholders.Speak Up culture.
AGAINST
The Board of Directors recommends that the shareholders vote AGAINST this proposal.
|
Merck & Co., Inc. 20232024 Proxy Statement
2024 Annual Meeting and Voting | | | | | |
97 | ||||||||
|
Date and Time: | Tuesday, May | |
Location: | Via Webcast at www.virtualshareholdermeeting.com/ | |
Record Date: | ||
We hope you will fully participate as a shareholder and exercise your right to vote. It is very important that you vote to play a part in the future of our Company. You do not need to attend the 2024 Annual Meeting of Shareholders to vote your shares.
Please cast your vote right away on all of the following proposals to ensure that your shares are represented:
| More information | Board’s recommendation | Broker discretionary voting allowed? | Votes required for approval | Abstentions and Broker Non-Votes | |||||||||||||
Proposal 1 |
Election of Directors |
Page |
FOR each Nominee |
No |
Majority of | Do not count for all proposals
(no effect) | |||||||||||
Proposal 2 |
Non-binding Advisory Vote to Approve the Compensation of our Named Executive Officers (Say-on-Pay) |
Page |
FOR |
No |
Majority of | ||||||||||||
Proposal 3 |
|
|
|
|
| ||||||||||||
| Ratification of Appointment of Independent Registered Public Accounting Firm for |
Page |
FOR |
Yes |
Majority of | ||||||||||||
Proposals 4, 5, |
Shareholder Proposals |
Pages |
AGAINST |
No |
Majority of | ||||||||||||
Why did I receive this Proxy Statement?
The Board of Directors is soliciting your proxy to vote at the 2024 Annual Meeting because you were a shareholder at the close of business on March 24, 2023,April 1, 2024, the record date, and are entitled to vote at the 2024 Annual Meeting.
This proxy statement and 2022 Annual Report onthe 2023 Form 10-K (the “Proxy Materials”), along with either a proxy card or a voting instruction form, or a Notice of Internet Availability of Proxy Materials, as applicable, are being distributed to shareholders beginning on April 3, 2023.11, 2024. This proxy statement summarizes the information you need to know to vote at the 2024 Annual Meeting. You do not need to attend the 2024 Annual Meeting to vote your shares.
What is the difference between holding shares as a shareholder of record and holding shares as a beneficial owner?
If your shares are registered directly in your name with Merck’sthe Company’s transfer agent, Equiniti Shareowner Services, you are considered the shareholder of record for those shares. The Proxy Materials and proxy card have been sent directly to you by Merck.the Company.
Merck & Co., Inc. 2023 Proxy Statement
|
|
104.99.
Merck & Co., Inc. 2024 Proxy Statement
| 98 | ç ç ç ç | Questions and Answers about the Annual Meeting and Voting |
What shares are included on the proxy card?
The shares on your proxy card represent shares registered in your name, as well as shares in the Merck Stock Investment Plan. However, the proxy card does not include shares held for participants in the Merck U.S. Savings Plan, MSD Employee Stock Purchase and Savings Plan and Merck Puerto Rico Employee Savings Plans and Security Plan. Instead, these participants will receive separate voting instruction cards covering these shares from plan trustees.
What constitutes a quorum?
As of the record date, 2,537,693,7762,533,028,189 shares of Merck common stock were issued and outstanding. Each share of common stock is entitled to one vote per share. A majority of the outstanding shares present at the 2024 Annual Meeting or represented by proxy constitutes a quorum for the transaction of business at the 2024 Annual Meeting. If you submit a properly executed proxy, then you will be considered part of the quorum.
How do I attend the 2024 Annual Meeting?
The 2024 Annual Meeting will be held in a solely virtual format, and all shareholders as of the record date, March 24, 2023,April 1, 2024, as well as guests are invited to attend. To attend the 2024 Annual Meeting, visit the online meeting platform at:
www.virtualshareholdermeeting.com/MRK2023.MRK2024.
Access to the meeting platform will begin at 8:45 a.m. on May 23, 202328, 2024 (Eastern Time).
If you are a shareholder as of the record date, March 24, 2023,April 1, 2024, you will be able to participate in the meeting by voting your shares and asking questions through the online meeting platform. To do so, visit the online meeting platform listed above and enter the 16-digit control number included on your proxy card, voting instruction form or Notice of Internet Availability of Proxy Materials when you visit the online meeting platform listed above.Materials. Guests may also access the 2024 Annual Meeting but may do so solely in listen-only mode. No control number is required for guests.
The meeting will include a question and answer session, and we will endeavor to answer as many questions submitted by shareholders as time permits. We reserve the right to edit profanity or other inappropriate language and to exclude questions regarding topics that are not pertinent to meeting matters or Company business. If we receive substantially similar questions, we may group them together and provide a single response to avoid repetition.
Asking questions:
You will have multiple opportunities to submit questions for the 2024 Annual Meeting.
| • | To submit a question before the 2024 Annual Meeting, visit www.proxyvote.com with your 16-digit control number and select the “Submit a Question” option. |
You can also submit a question via the online platform live during the 2024 Annual Meeting.
Whether or not you plan to attend the 2024 Annual Meeting, we urge you to vote and submit your proxy in advance by one of the advance voting methods described in the “How do I vote?” section below.
If you encounter any technical difficulties with the meeting platform on the date of the 2024 Annual Meeting, technical support will be available during this time and will remain available until the virtual 2024 Annual Meeting has ended.
Merck & Co., Inc. 2023 Proxy Statement
|
|
If you are a shareholder of record, you may vote using any of the following methods:
| • | Proxy card. Be sure to complete, sign and date the card and return it in the prepaid envelope. |
| • | Via the internet. You may vote online at www.proxyvote.com. You will need the 16-digit control number on the proxy card or the Notice of Internet Availability of Proxy Materials. |
| • | By telephone. You may vote by calling 1-800-690-6903 (toll free). The telephone voting facilities will close at 11:59 p.m. Eastern Time on May |
Merck & Co., Inc. 2024 Proxy Statement
Questions and Answers about the Annual Meeting and Voting | ÷ ÷ ÷ ÷ | 99 |
| • | By QR code. You may vote by scanning with your mobile device the QR code included on your proxy card or Notice of Internet Availability of Proxy Materials. You may also vote by scanning the QR code on page 1, |
| • | At the 2024 Annual Meeting. All shareholders may vote at the 2024 Annual Meeting. Please see “How do I attend the 2024 Annual Meeting?” above. |
If you are a beneficial owner of shares, you may vote by following the voting instructions provided by your broker, bank or nominee. You may also vote at the 2024 Annual Meeting.
If you own | How to vote at the 2024 Annual Meeting | |
in your name, you are a shareholder of record and | You may vote at the virtual meeting by visiting www.virtualshareholdermeeting.com/ | |
through a broker, bank, or nominee, you are a beneficial owner of shares and | You may vote at the virtual meeting by visiting www.virtualshareholdermeeting.com/ | |
What can I do if I change my mind after I vote my shares?
If you are a shareholder of record, you may revoke your proxy at any time before it is voted at the 2024 Annual Meeting by:
sending written notice of revocation to the Secretary of the Company;
submitting a revised proxy by telephone, internet or paper ballot after the date of the revoked proxy; or
attending the 2024 Annual Meeting and voting.
If you are a beneficial owner of shares, you may submit new voting instructions by contacting your broker, bank or nominee. You may also vote during the 2024 Annual Meeting.
Will my votes be confidential?
Yes. Only the personal information necessary to enable proxy execution, such as control number or shareholder signature, is collected on the paper or online proxy cards.
All shareholder proxies and ballots that identify individual shareholders are kept confidential and are not disclosed except as required by law.
Who will count the vote?
Representatives of First Coast Results will tabulate the votes and act as inspectors of election.
Voting information for beneficial owners
If you hold your shares through a broker, bank or nominee, you are considered the beneficial owner of those shares, but not the shareholder of record. As a beneficial owner, you will receive voting instructions from your broker, bank or nominee and you must communicate your voting decisions to that particular institution (not the Company) by using the voting instruction forminstructions that the institution provides to you. You may also vote your shares via telephone or the internet by following the specific instructions the institution provides to you for that purpose.
Merck & Co., Inc. 2023 Proxy Statement
|
|
the election of directors and other mattersany matter to be considered at the 2024 Annual Meeting (exceptexcept on ratification of the appointment of PricewaterhouseCoopers LLP as the independent registered public accounting firm for 2023).2024. If you do not provide voting instructions, your shares will not be voted on any proposalmatter other than the ratification of the auditors. This is called a “broker non-vote.”
For your vote to be counted, you must communicate your voting decisions to your broker, bank or nominee before the date of the 2024 Annual Meeting.
Merck & Co., Inc. 2024 Proxy Statement
| 100 | ç ç ç ç | Questions and Answers about the Annual Meeting and Voting |
What if I am a shareholder of record and I return my proxy card but do not provide voting instructions?
If you are a shareholder of record and you return your signed proxy card but do not indicate your voting preferences, the individuals named in the proxy card will vote on your behalf as follows:
| • | FOR the election as Directors of each of the |
| • | FOR the approval of the compensation of our Named Executive Officers by a non-binding advisory vote; |
| • | FOR the |
|
| • | AGAINST the shareholder proposals. |
What if I am a plan participant and do not provide voting instructions?
If voting instructions are not received for shares held in the Merck U.S. Savings Plan or the MSD Employee Stock Purchase and Savings Plan, those shares will not be voted. If voting instructions are not received from participants in the Merck Puerto Rico Employee Savings and Security Plan, the plan trustee will vote the shares you hold in the same proportion as the shares held in these plans for which voting instructions were timely received.
What is “householding” and how does it affect me?
Merck has adopted the process called “householding” for mailing the Proxy Materials and Notice of Internet Availability of Proxy Materials in order to reduce printing costs and postage fees. Householding means that shareholders who share the same last name and address will receive only one copy of the Proxy Materials or Notice of Internet Availability of Proxy Materials, as applicable, unless we receive contrary instructions from any shareholder at that address. Merck will continue to mail a proxy card to each shareholder of record.
Can I access the Proxy Materials on the internet instead of receiving paper copies?
The Proxy Materials are available on Merck’s website at www.merck.com/investor-relations/financial-information/. If you are a shareholder of record, you may choose to stop receiving paper copies of Proxy Materials in the mail by following the instructions given while you vote by telephone or through the internet. If you choose to access future Proxy Materials on the internet, you will receive an email message next year that will provide a link to those documents. Your choice will remain in effect until you advise us otherwise.
If you are a beneficial owner of shares, please refer to the information provided by your broker, bank or nominee for instructions on how to elect to access future Proxy Materials electronically. Most beneficial owners of shares who elect electronic access will receive an email message next year containing the URL for accessing the Proxy Materials.
If you prefer to receive multiple copies of the Proxy Materials or Notice of Internet Availability of Proxy Materials, as applicable, at the same address for the 20232024 Annual Meeting or for future annual meetings, additional copies will be provided promptly upon written or oral request. If you are a shareholder of record, you may contact us by writing to EqunitiEquiniti Shareowner Services, P.O. Box 64874, St. Paul, MN 55164-0874 or calling 1-800-522-9114. The request should include your account number. Eligible shareholders of record receiving multiple copies of the Proxy Materials or Notice of Internet Availability of Proxy Materials, as applicable, can request householding by contacting Merck in the same manner.
Merck & Co., Inc. 2023 Proxy Statement
|
|
Where can I find the results of the 2024 Annual Meeting?
We will post the final voting results the Friday following the 2024 Annual Meeting on our website www.merck.com under “Investors.” We also intend to disclose the final voting results on Form 8-K within four business days of the 2024 Annual Meeting.
Merck & Co., Inc. 2024 Proxy Statement
Questions and Answers about the Annual Meeting and Voting | ÷ ÷ ÷ ÷ | 101 |
Where can I find the 2022 Annual Report on2023 Form 10-K?
The 2022 Annual Report on Form2023 10-K is available on Merck’s website at www.merck.com/investor-relations/financial-information/.
In addition, we will provide without charge a copy of the 2022 Annual Report on Form2023 10-K, including financial statements and schedules, upon the written request of any shareholder to the Office of the Secretary, Merck & Co., Inc., 126 East Lincoln Avenue, Rahway, N.J. 07065 U.S.A. Shareholders may also email the Office of the Secretary at office.secretary@merck.com to make such request.
How much did this proxy solicitation cost?
The Company retained Morrow Sodali LLC to assist in the distribution of the Proxy Materials and solicitation of votes for $20,000, plus reasonable out-of-pocket expenses. Employees, officers and Directors of the Company also may solicit proxies by telephone or in-person meetings. We will pay the solicitation costs and reimburse brokerage houses and other custodians, nominees and fiduciaries for their reasonable out-of-pocket expenses for forwarding proxy and solicitation materials to shareholders.
Merck & Co., Inc. 20232024 Proxy Statement
| 102 | ç ç ç ç |
|
Shareholder Proposals and Director Nominations for the 20242025 Annual Meeting of Shareholders
Deadline for Receipt of Shareholder Proposals for Inclusion in the Proxy Materials for the 20242025 Annual Meeting of Shareholders
In order to be considered for inclusion in next year’s proxy statement in accordance with SEC Rule 14a-8, shareholder proposals must be submitted in writing to the address shown below and received by the close of business, Eastern Time, on December 5, 2023.11, 2024.
Director Nominees for Inclusion in the Proxy Materials for the 20242025 Annual Meeting of Shareholders (Proxy Access)
Shareholders who intend to nominate a person for election as director under the proxy access provision in our By-Laws for inclusion in our Proxy Materials must comply with the provisions of, and provide notice to us in accordance with, Section 3 of Article II of our By-Laws. That section sets forth shareholder eligibility requirements and other procedures that must be followed and the information that must be provided in order for an eligible shareholder to have included in our Proxy Materials up to two Director nominees. For the 20242025 Annual Meeting of Shareholders, we must receive the required notice between November 5, 2023,11, 2024, and December 5, 2023,11, 2024, at the address shown below. Such notice must include the information required by our By-Laws, which are available on our website at www.merck.com/company-overview/leadership/board-of-directors/.
Shareholder Proposals, Director Nominations, and Other Business to be Brought Before the 20242025 Annual Meeting of Shareholders
Any shareholder who wishes to present proposals, director nominations or other business for consideration directly at the 20242025 Annual Meeting of Shareholders but does not intend to have such proposals or nominations included in Merck’s Proxy Materials must submit the proposal or nomination in writing to the address shown below so that it is received between December 25, 2023,29, 2024, and January 24, 2024.28, 2025. However, in the event that the date of the 20242025 Annual Meeting of Shareholders is more than 30 days earlier or later than the anniversary date of this year’s annual meeting, such notice must be so received not later than the close of business on the later of the 120th day prior to the 20242025 Annual Meeting of Shareholders or the 10th day following the day on which a public announcement of the date of the 20242025 Annual Meeting of Shareholders is first made.
Written notice of proposals or other business for consideration must contain the information specified in Article I, Section 6 of our By-Laws. Written notice of nomination must contain the information set forth in Article II, Section 2 of our By-Laws. Our By-Laws are available online at www.merck.com/company-overview/leadership/board-of-directors/ or upon request to the Office of the Secretary.
This written notice requirement does not apply to shareholder proposals properly submitted for inclusion in our proxy statement in accordance with the rules of the SEC and shareholder nominations of director candidates.
ADDRESS TO CONTACT THE COMPANY
Any notice required to be sent to the Company as described above should be emailed to office.secretary@merck.com, or
mailed to the Office of the Secretary, Merck & Co., Inc., 126 East Lincoln Avenue, Rahway, N.J. 07065 U.S.A.
Merck & Co., Inc. 20232024 Proxy Statement
| ÷ ÷ ÷ ÷ | 103 |
Forward-Looking Statements
This Proxy MaterialStatement contains “forward-looking statements” as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements are based on management’s current expectations and involve risks and uncertainties, which may cause results to differ materially from those set forth in the statements. The forward-looking statements may include without limitation statements regarding growth strategy, financial results, product development,approvals, product potential, development programs, environmental or financial performance.other sustainability initiatives. No forward-looking statement can be guaranteed and actual results may differ materially from those projected. Merck undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise. Forward-looking statements should be evaluated together with the many uncertainties that affect Merck’s business, particularly those mentioned in the risk factors and cautionary statements in Item 1A of Merck’s Annual Report on Form 10-K for the year ended December 31, 2022,2023 10-K, and in its periodic reports on Form 10-Q and current reports on Form 8-K, if any, which we incorporate by reference.
Other Matters
The Board of Directors is not aware of any other matters to come before the meeting. However, if any other matters properly come before the meeting, it is the intention of the persons named in the enclosed proxy to vote said proxy in accordance with their judgment in such matters.
Merck & Co., Inc.
April 3, 202311, 2024
Merck & Co., Inc. 20232024 Proxy Statement
104 | | | | | |
|
| |||||||
Appendix A—Non-GAAP Income and Non-GAAP EPS |
Merckthe Company is providing because management believes this information enhances investors’ understanding of the Company’s results since management uses non-GAAP measures to assess performance. Non-GAAP income and non-GAAP EPS exclude certain items because of the nature of these items and the impact that they have on the analysis of underlying business performance and trends. The excluded items (which should not be considered non-recurring) consist of acquisitionacquisition- and divestiture-related costs, restructuring costs, income and losses from investments in equity securities, and certain other items. These excluded items are significant components in understanding and assessing financial performance.
Non-GAAP income and non-GAAP EPS are important internal measures for the Company. Senior management receives a monthly analysis of operating results that includes a non-GAAP EPS metric. Management uses non-GAAP measures internally for planning and forecasting purposes and to measure the performance of the Company along with other metrics. In addition, annual employee compensation, including senior management’s annual compensation, is derived in part using a non-GAAP pretax income metric. Since non-GAAP income and non-GAAP EPS are not measures determined in accordance with GAAP, they have no standardized meaning prescribed by GAAP and, therefore, may not be comparable to the calculation of similar measures of other companies. The information on non-GAAP income and non-GAAP EPS should be considered in addition to, but not as a substitute for or superior to, net income and EPS prepared in accordance with GAAP.
In 2022, the Company changed the treatment of certain items for purposes of its non-GAAP reporting. Historically, Merck’s non-GAAP results excluded expenses for upfront and pre-approval milestone payments related to collaborations and licensing agreements, as well as charges related to pre-approval assets obtained in transactions accounted for as asset acquisitions, to the extent the charges were considered by the Company to be significant to the results of a particular period (as well as any related adjustments recorded in a subsequent period). Merck’s non-GAAP results no longer exclude charges related to these items. Prior periods have been recast to conform to the current presentation.
A reconciliation between GAAP financial measures and non-GAAP financial measures (from continuing operations) is as follows:
($ in millions except per share amounts) | Year Ended
| ||||||
Income from continuing operations before taxes as reported under GAAP | |||||||
Increase (decrease) for excluded items: | |||||||
Acquisition and divestiture-related costs(1) | |||||||
Restructuring costs | |||||||
| ) | ||||||
Other items: | |||||||
Charge for Zetia antitrust litigation settlements | 573 | ||||||
Non-GAAP income from continuing operations before taxes | |||||||
Taxes on income from continuing operations as reported under GAAP | |||||||
Estimated tax benefit on excluded items(2) | |||||||
Non-GAAP taxes on income from continuing operations | |||||||
Non-GAAP net income from continuing operations | |||||||
Less: Net income attributable to noncontrolling interests as reported under GAAP | |||||||
Non-GAAP net income from continuing operations attributable to Merck & Co., Inc. | |||||||
EPS assuming dilution from continuing operations as reported under GAAP(3) | |||||||
EPS difference | |||||||
Non-GAAP EPS assuming dilution from continuing operations(3) | |||||||
| (1) | Amount in |
| (2) | The estimated tax impact on the excluded items is determined by applying the statutory rate of the originating territory of the non-GAAP adjustments. |
| (3) | GAAP and non-GAAP EPS were negatively affected in 2023 by $6.21 of charges for certain upfront and pre-approval milestone payments related to collaborations and licensing agreements, as well as charges related to pre-approval assets obtained in transactions accounted for as asset acquisitions. |
| (1) | “GAAP” is defined as generally accepted accounting principles in the United States. |
Merck & Co., Inc.2023 2024 Proxy Statement
Appendix A Non-GAAP Income and Non-GAAP EPS | ÷ ÷ ÷ ÷ | 105 |
Non-GAAP income and non-GAAP EPS exclude the impact of certain amounts recorded in connection with acquisitions and divestitures of businesses. These amounts include the amortization of intangible assets and amortization of purchase accounting adjustments to inventories, as well as intangible asset impairment charges, and expense or income related to changes in the estimated fair value measurement of liabilities for contingent consideration. Also excluded are integration, transaction, and certain other costs associated with acquisitions and divestitures of businesses.divestitures. Non-GAAP income and non-GAAP EPS also exclude amortization of intangible assets related to collaborations and licensing arrangements.
Restructuring Costs
Non-GAAP income and non-GAAP EPS exclude costs related to restructuring actions (see Note 6 to the Company’s Consolidated Financial Statements in the Annual Report on Form 10-K for the year ended December 31, 2022)2023 10-K). These amounts include employee separation costs and accelerated depreciation associated with facilities to be closed or divested. Accelerated depreciation costs represent the difference between the depreciation expense to be recognized over the revised useful life of the asset, based upon the anticipated date the site will be closed or divested or the equipment disposed of, and depreciation expense as determined utilizing the useful life prior to the restructuring actions. Restructuring costs also include asset abandonment, facility shut-down and other related costs, as well as employee-related costs such as curtailment, settlement and termination charges associated with pension and other postretirement benefit plans and share-based compensation costs.
Income and Losses from Investments in Equity Securities
Non-GAAP income and non-GAAP EPS exclude realized and unrealized gains and losses from investments in equity securities either owned directly or through ownership interests in investment funds.
Certain Other Items
Non-GAAP income and non-GAAP EPS exclude certain other items. These items are adjusted for after evaluating them on an individual basis, considering their quantitative and qualitative aspects. Typically, these consist of items that are unusual in nature, significant to the results of a particular period or not indicative of future operating results. Excluded from non-GAAP income and non-GAAP EPS are chargesis a charge related to settlements with certain plaintiffs in the discontinuation ofZetia antitrust litigation (see COVID-19 development programs (see Note 4 11 to the Company’s Consolidated Financial Statements in the Annual Report on Form 10-K for the year ended December 31, 2022), as well as2023 10-K) and a net tax benefit related to the settlement of certain federal income tax matters (see Note 16 to the Company’s Consolidated Financial Statements in the Annual Report on Form 10-K for the year ended December 31, 2022), and an adjustment to tax benefits recorded in conjunction with the 2015 acquisition of Cubist Pharmaceuticals, Inc.2023 10-K).
Merck & Co., Inc. 20232024 Proxy Statement
106 | | | | | |
|
| |||||||
Appendix B—Explanation of Adjustments to Non-GAAP Results For Incentive Plans |
Program | Financial Metric | Weighting of Component | Definition | Adjustments | |||||||||
  |
Pipeline |
20% |
The Company’s Research and Development goals for the
|
No Adjustments | |||||||||
|
|
The Company’s | No Adjustments | ||||||||||
Revenue | 35% |
Excludes charges or items from the measurement of performance relating to (1) the impact of significant
| |||||||||||
Pre-Tax |
|
The Company’s target non-GAAP income before taxes |
Excludes charges or items from the measurement of performance relating to (1) restructurings, discontinued operations, purchase accounting items, merger-related costs, the impact of significant acquisitions and/or divestitures, extraordinary items and other unusual or non-recurring charges and/ or events; (2) an event either not directly related to Company operations or not reasonably within the control of Company management; (3) fluctuations in
| ||||||||||
  |
Earnings (or EPS) |
33% |
The Company’s after-tax Non-GAAP net income (attributable to the Company) divided by total shares outstanding assuming dilution
|
All of the adjustments listed for “Pre-Tax Income” above, as well as (1) the impact of | |||||||||
Relative |
67% |
The comparison of the Company’s
|
No Adjustments |
* The PSU program design discussed above refers to the 2020-20222021-2023 performance period.period that was designed this way due to the complexities associated with disentangling the Company’s Organon business from a multi-year financial plan. Refer to page 5657 for PSU program designs relating to the 2020-20222022-2024 and 2021-20232023-2025 performance periods as a result of the Organon spin-off.periods.
Merck & Co., Inc.2023 2024 Proxy Statement
Environmental, Social and Governance (ESG)Sustainability Highlights
External Recognition for Our ESGSustainability Performance
We are proud of the external recognition we have received for our ESG-relatedsustainability-related initiatives and performance.
Responsible Companies list, and #1 in the healthcare and life sciences sector, which is the fourth year in a row we have been recognized (2024) | Wall Street Journal Ranked #10 on its list of the 250 best-managed publicly traded U.S. companies (2023) | JUST Capital Ranked #25 overall and #1 in the pharmaceuticals and biotech sector on the
| ||
Barron’s
Recognized on Barron’s Top 100 Most Sustainable Companies
|
#1 in the
| |||
Ranked #7 overall and #1 in sector in the
| ||||
ESGSustainability Resources
In addition to our annual ESG ProgressImpact Report, there are resources on our corporate website that demonstrate our commitment to ESGsustainability and its value to drive sustainable impact to society and to our business. To access these resources, please visit the following pagesbelow sites on our corporate website:website.
| • |
|
| • |
|
| • | Transparency Disclosures at http://www.merck.com/company-overview/ |
| • | Policies & Positions at http://www.merck.com/company-overview/policies-and-positions/ |
| • | Impact Investing at http://www.merck.com/company-overview/ |
| • | Code of Conduct & Compliance at http://www.merck.com/company-overview/culture-and-values/code-of-conduct/ |
For more information about our approach to ESG,sustainability, please visit our 2021/2022 ESG Progress2022/2023 Impact Report at http://www.merck.com/company-overview/esg/sustainability/.


MERCK & CO., INC.
126 EAST LINCOLN AVENUE
RAHWAY, NJ 07065

SCAN TO VIEW MATERIALS & VOTE w ELECTRONIC DELIVERY OF FUTURE PROXY MATERIALS
If you would like to reduce the costs incurred by our company in mailing proxy materials, you can consent to receiving all future proxy statements, proxy cards and annual reports electronically via e-mail or the Internet. To sign up for electronic delivery, please follow the instructions below to vote using the Internet and, when prompted, indicate that you agree to receive or access proxy materials electronically in future years.
VOTE BY INTERNET
Before The Meeting - Go towww.proxyvote.com or scan the QR Barcode above
Use the Internet to transmit your voting instructions and for electronic delivery of information up until 11:59 p.m. Eastern Time on May 22, 2023.27, 2024. Have your proxy card in hand when you access the website and follow the instructions to obtain your records and to create an electronic voting instruction form.
During The Meeting - Meeting—Go to www.virtualshareholdermeeting.com/MRK2023
MRK2024 You may attend the meeting via the Internet and vote during the meeting. Have the information that is printed in the box marked by the arrow available and follow the instructions.
VOTE BY PHONE - PHONE—1-800-690-6903
Use any touch-tone telephone to transmit your voting instructions up until 11:59 p.m. Eastern Time on May 22, 2023.27, 2024. Have your proxy card in hand when you call and then follow the instructions.
VOTE BY MAIL
Mark, sign and date your proxy card and return it in the postage-paid envelope we have provided or return it to Merck & Co, Inc., c/o Broadridge, 51 Mercedes Way, Edgewood, NY 11717.
TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS:
V10971-P88229-Z84479 V37850-P05716-Z86974 KEEP THIS PORTION FOR YOUR RECORDS
— — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — —
DETACH AND RETURN THIS PORTION ONLY
THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED.
DETACH AND RETURN THIS PORTION ONLY MERCK & CO., INC.
The Board of Directors recommends you vote FOR each of the following Nominees: 1. Election of Directors For Against Abstain Nominees: 1a. Douglas M. Baker, Jr. ! ! ! 1b. Mary Ellen Coe ! ! ! The Board of Directors recommends you vote FOR proposals 2 and 3: For Against Abstain 1c. Pamela J. Craig ! ! ! 2. Non-binding advisory vote to approve the compensation of our ! ! ! named executive officers. 3. Ratification of the appointment of the Company’s independent 1d. Robert M. Davis ! ! ! registered public accounting firm for 2024. ! ! ! 1e. Thomas H. Glocer ! ! ! The Board of Directors recommends you vote AGAINST proposals For Against Abstain 4, 5 and 6. 4. Shareholder proposal regarding a shareholder right to act by written 1f. Risa J. Lavizzo-Mourey, M.D. ! ! ! ! ! ! consent. 1g. Stephen L. Mayo, Ph.D. ! ! ! 5. Shareholder proposal regarding a government censorship ! ! ! transparency report. 1h. Paul B. Rothman, M.D. ! ! ! 6. Shareholder proposal regarding a report on respecting workforce ! ! ! civil liberties. 1i. Patricia F. Russo ! ! ! 1j. Christine E. Seidman, M.D. ! ! ! 1k. Inge G. Thulin ! ! ! 1l. Kathy J. Warden ! ! ! Signature [PLEASE SIGN WITHIN BOX] Date Signature (Joint Owners) Date |
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
|
|
|
| |||||||

Merck & Co., Inc.
Annual Meeting of Shareholders
Tuesday, May 23, 2023,28, 2024, at 9:00 a.m. (Eastern Time)
via Webcast at
www.virtualshareholdermeeting.com/MRK2023*
MRK2024* Important Notice Regarding the Availability of Proxy Materials for the Shareholder Meeting to be Held on May 23, 2023:
28, 2024: The Notice and Proxy Statement and Annual Report on Form 10-K are available at www.proxyvote.com.
*We *We have adopted a virtual format for the 20232024 Annual Meeting of Shareholders to provide a convenient and consistent experience to all shareholders regardless of location.
— — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — —
V10972-P88229-Z84479
 |
|
V37851-P05716-Z86974 THIS PROXY IS SOLICITED ON BEHALF OF THE BOARD OF DIRECTORS The undersigned hereby appoints ROBERT M. DAVIS, JENNIFER ZACHARY and KELLY GREZ as Proxies, each with the power to appoint his/her substitute, and hereby authorizes them to represent and to vote ALL of the stock of MERCK & CO., INC. standing in the name of the undersigned at the ANNUAL MEETING OF SHAREHOLDERS to be held at 9:00 a.m. (Eastern Time) on May 23, 2023,28, 2024, and at all adjournments or postponements thereof, upon the matters set forth on the reverse side, as designated, and upon such other matters as may properly come before the meeting. This card also provides voting instructions for shares held for the account of the undersigned in the Merck Stock Investment Plan. Any prior proxy or voting instructions are hereby revoked.
The shares represented by this proxy will be voted as directed by the shareholder and in accordance with the judgment of the Proxies upon any other matter that may properly come before the meeting and any adjournment or postponement thereof. If no specification is made, the shares will be voted FOR each nominee in Item 1, FOR Items 2 and 4, ONE YEAR for Item 3, and AGAINST Items 4, 5 6, 7, 8, 9 and 10.
6. IF YOU VOTE BY TELEPHONE OR BY INTERNET, DO NOT MAIL THIS PROXY CARD. THE TELEPHONE AND INTERNET VOTING FACILITIES WILL CLOSE AT 11:59 P.M. ON MAY 22, 2023.
27, 2024. Please complete, sign, date and return the Proxy Card promptly using the enclosed envelope.
(Continued, (Continued, and to be signed and dated on the reverse side.)




 Our business development strategy is helping position Merck for growth well into the next decade
Our business development strategy is helping position Merck for growth well into the next decade













































 Allow Directors and management employees, including officers, to engage in transactions involving short sales, publicly traded options, hedging or pledging of
Allow Directors and management employees, including officers, to engage in transactions involving short sales, publicly traded options, hedging or pledging of